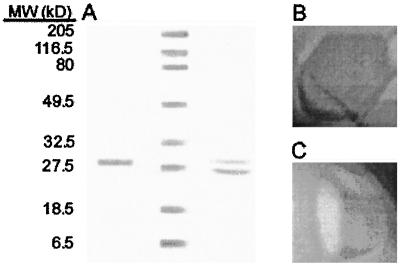
Figure 7.
Purification and antifreeze activity of CHT46 produced by expressing OmpA/His6/CHT46 in E. coli JM105 cells. A, As shown in the left lane, OmpA-His6-CHT46 (1.2 μg) was purified from cell lysates, solubilized, and separated on a SDS-polyacrylamide gel (12%, w/v) stained with Coomassie Blue. The right-hand lane shows the presence of CHT46 after thrombin cleavage of OmpA-His6-CHT46. The positions of Bio-Rad broad range prestained molecular mass protein standards (center lane) are shown on the left. B, After cleavage of the OmpA leader and His-tag, hexagonally shaped ice crystals grew during freezing of CHT46 solutions containing 0.06 mg of total soluble protein mL−1 (ice crystal shown with c-axis perpendicular to the plane of the page). C, After concentrating the solution containing CHT46 to 1.35 mg of total soluble protein mL−1, the ice crystals grew to form hexagonally shaped columns (in the crystal shown, the c-axis is parallel to the plane of the page). The increased c-axis growth of the ice crystal indicates a greater amount of antifreeze activity.