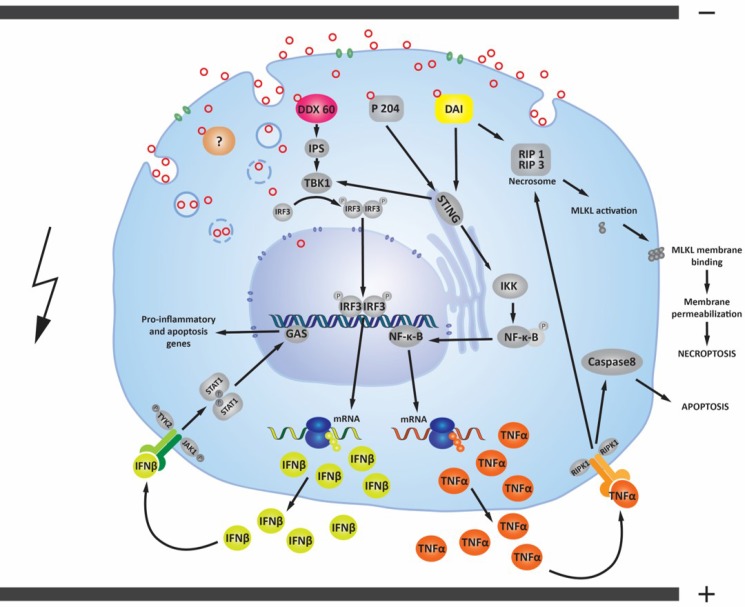
Figure 6. Possible signaling pathways activated by pDNA electrotransfer of tumor cells.
After electrotransfer, pDNA (red circles) primarily enters the cell through endocytosis (blue circles). Plasmid DNA must undergo endosomal escape to enter the cytosol. Endosomal escape can occur early in the endocytosis process or later, before pDNA enters the nucleus [21, 26]. pDNA may possibly enter the cytosol directly via electropores formed in cell membrane [28]. The presence of pDNA inside the cytosol of different tumor cell lines is associated with the upregulation of cytosolic DNA sensors DDX60, DAI/ZBP1 and the p204 [44, 73, 74]. If these cytosolic DNA sensors are activated by binding pDNA, the transcription factor interferon regulator factor 3 (IRF3) is transported from the cytosol to the nucleus to induce transcription of IFNβ and other genes [29, 47, 82, 98]. The upregulated and secreted IFNβ protein may bind to its cell-surface receptor(s) in an autocrine or paracrine fashion, leading to transcription of pro-inflammatory and apoptotic genes. The DNA sensors p204 and DAI/ZBP1 can activate a signaling pathway which leads to the nuclear translocation of the transcription factor NF-ĸB and consequently to transcription of pro-inflammatory cytokines, such as TNFα, which is secreted from the cell [29, 47, 80, 81, 99]. TNFα may bind to cell-surface receptors, which leads to the activation of two different death mechanisms (apoptosis or necroptosis) [90]. Another signaling pathway of the cytosolic DNA sensor DAI/ZBP1 leads directly to necroptosis through MLKL activation [100]. Cytosolic DNA sensors and their signaling pathways described here are present and upregulated in mouse TS/A adenocarcinoma cells, mouse WEHI 164 fibrosarcoma cells, and mouse B16F10 melanoma cells [25]. Other DNA sensors, whether upregulated, not upregulated, or as yet undiscovered (labeled with “?”) may also influence these signaling pathways.