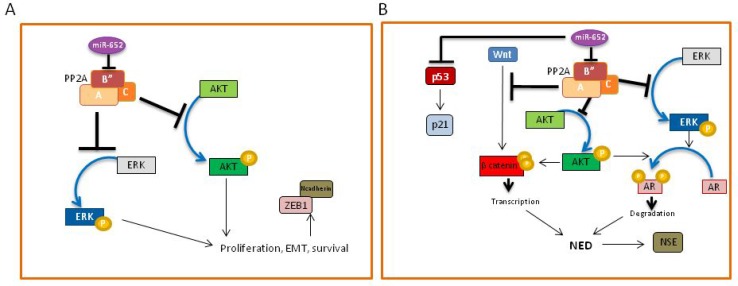
Figure 8.
(A) Model of miR-652-mediated mechanism of EMT in PC3 cells. The presence of miR-652 results in downregulation of the PPP2R3A regulatory subunit (B”) of PP2A, resulting in inhibition of PP2A activity. This leads to the abrogation of PP2A-mediated inhibition of AKT and ERK kinases, resulting in their phosphorylation and activation. The result is increased cell growth, survival and EMT, with the downregulation of Ecadherin, and upregulation of the mesenchymal markers, N-cadherin and ZEB1. (B) Model of miR-652-mediated neuroendocrine differentiation of LNCaP cells. The presence of miR-652 results in downregulation of the PPP2R3A regulatory subunit (B”) of PP2A, resulting in inhibition of PP2A activity. This leads to the abrogation of PP2A-mediated inhibition of AKT and ERK kinases, resulting in their phosphorylation and activation. Activated AKT and ERK are both able to phosphorylate the androgen receptor (AR), resulting in its degradation. PP2A inhibition by miR-652 also releases the Wnt pathway from inhibition, resulting in the phosphorylation (Ser552) and activation of β-catenin, which allows its translocation to the nucleus to impact gene transcription. Activated β-catenin, together with degradation of AR results in transcription of proteins required for neuroendocrine differentiation, specifically, NSE. MiR-652 may also directly inhibit p53, leading to p21 inactivation. Together, this would allow the cells to undergo cell cycle progression.