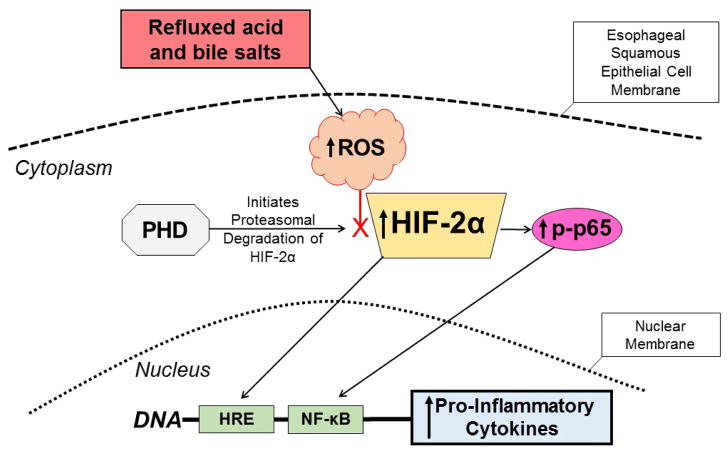
Figure 1.
Proposed mechanism for the pathogenesis of reflux esophagitis. Esophageal squamous epithelial cells exposed to refluxed acid and bile salts generate intracellular ROS that decrease the activity of PHD, the enzyme that initiates proteasomal degradation of HIF-2α. The decreased PHD activity stabilizes HIF-2α and enables it to accumulate in the cytoplasm. The stabilized HIF-2α then can translocate to the nucleus to induce transcription of pro-inflammatory cytokine genes that have hypoxia responsive elements (HREs) in their promoter regions. The stabilized HIF-2α in the cytoplasm also can increase levels of phospho-p65, enabling it to translocate to the nucleus and bind the NF-κB gene promoter, which further stimulates production of the pro-inflammatory cytokines that mediate the development of reflux esophagitis. ROS=reactive oxygen species, PHD=prolyl hydroxylase, HIF=hypoxia inducible factor, HRE= hypoxia responsive element, NF-κB=nuclear factor κB.