Abstract
The intrinsic cardiac nervous system modulates cardiac function by acting as an integration site for regulating autonomic efferent cardiac output. This intrinsic system is proposed to be composed of a short cardio-cardiac feedback control loop within the cardiac innervation hierarchy. For example, electrophysiological studies have postulated the presence of sensory neurons in intrinsic cardiac ganglia for regional cardiac control. There is still a knowledge gap, however, about the anatomical location and neurochemical phenotype of sensory neurons inside intrinsic cardiac ganglia. In the present study, rat intrinsic cardiac ganglia neurons were characterized neurochemically with immunohistochemistry using glutamatergic markers: vesicular glutamate transporters 1 and 2 (VGLUT1; VGLUT2), and glutaminase (GLS), the enzyme essential for glutamate production. Glutamatergic neurons (VGLUT1/VGLUT2/GLS) in the ICG that have axons to the ventricles were identified by retrograde tracing of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) injected in the ventricular wall. Co-labeling of VGLUT1, VGLUT2, and GLS with the vesicular acetylcholine transporter (VAChT) was used to evaluate the relationship between post-ganglionic autonomic neurons and glutamatergic neurons. Sequential labeling of VGLUT1 and VGLUT2 in adjacent tissue sections was used to evaluate the co-localization of VGLUT1 and VGLUT2 in ICG neurons. Our studies yielded the following results: (1) intrinsic cardiac ganglia contain glutamatergic neurons with GLS for glutamate production and VGLUT1 and 2 for transport of glutamate into synaptic vesicles; (2) atrial intrinsic cardiac ganglia contain neurons that project to ventricle walls and these neurons are glutamatergic; (3) many glutamatergic ICG neurons also were cholinergic, expressing VAChT. (4) VGLUT1 and VGLUT2 co-localization occurred in ICG neurons with variation of their protein expression level. Investigation of both glutamatergic and cholinergic ICG neurons could help in better understanding the function of the intrinsic cardiac nervous system.
Keywords: intrinsic cardiac ganglia, glutaminase, vesicular glutamate transporter 1 & 2, vesicular acetylcholine transporter, wheat germ agglutinin-horseradish peroxidase, sensory neurons
Introduction
The intrinsic cardiac ganglia (ICG) consist of groups of ganglion neurons that reside on the dorsal surface of the atrium. This ganglionic system is considered to be composed of a short cardio-cardiac neuronal feedback loop that functions in modulating regional cardiodynamics. Within this system, three major neuronal components have been proposed: autonomic (parasympathetic and sympathetic) efferent neurons, local circuit neurons, and sensory neurons (Armour, 1999, 2004, Kukanova and Mravec, 2006, Armour, 2008). The autonomic efferent neurons within the ICG receive sympathetic and parasympathetic preganglionic information from higher neuronal hierarchy to modulate heart rate, conduction velocity and contractility (Ardell et al., 1988, Gatti et al., 1995, Cheng et al., 1999, Cheng and Powley, 2000, Armour, 2008). Efferent neurons project to cardiac tissue including the sinoatrial (SA) node, atrioventricular (AV) node and contractile tissues (e.g., papillary muscle). Although often thought of as relay ganglia, the ICG also were proposed to contain both interneurons and sensory neurons. Local mechanical and chemical stimuli on the cardiac ventricles and major vessels activate ICG neurons and the information is transmitted to local circuit neurons and/or autonomic efferent neurons for adjusting their neuronal activity (Ardell et al., 1991, Armour et al., 1997, Thompson et al., 2000, Armour, 2008).
In earlier electrophysiology studies, “sensory-like” cells were described in the ICG of different species and were characterized by prolonged after hyperpolarization (AHP) and inward rectification at slightly negative membrane potentials. These membrane electrical properties resemble the electrophysiology pattern of primary sensory neurons in dorsal root ganglia (DRG)(Selyanko, 1992, Edwards et al., 1995). In histology studies, the presence of bipolar or pseudounipolar neurons in ICG resembles primary sensory neurons in DRG and nodose ganglion (NG)(Pauza et al., 1997, Moravec and Moravec, 1998). Moreover, ventricular epicardial mechanical stimulation induces neuronal activation in ICG (Horackova et al., 1999, Thompson et al., 2000). Chemicals, such as substance P, ATP, neuropeptide Y, bradykinin and adenosine, change the neuronal activity within the ICG when applied to the ventricular cardiac surface (Armour et al., 1998, Armour, 1999, Thompson et al., 2000). These studies indicate that some ICG neurons possess sensory properties and are capable of transmitting information about regional cardiac function and milieu. An understanding, however, of the neurochemical phenotype of these potential sensory neurons and their anatomical location inside the ICG is still lacking.
Neurochemical and electrophysiological studies have determined that DRG primary sensory neurons have a glutamatergic neurotransmitter phenotype (Miller et al., 2011). In the current study, we propose that ICG primary sensory neurons use glutamate as their neurotransmitter. In order to evaluate this proposal, we first investigated if glutamatergic neurons were present within ICG using immunohistochemistry for glutaminase (GLS), synthetic enzyme for glutamate, and vesicular glutamate transporter 1 and 2 (VGLUT1 and 2). Secondly, WGA-HRP retrograde tracing from the ventricular wall was used coupled with immunohistochemistry for GLS and VGLUT 1, 2 to detect potential sensory ICG neurons. Thirdly, to compare ICG neurons with glutamatergic vs. cholinergic phenotype, we co-stained with immunohistochemistry for vesicular acetylcholine transporter (VAChT) and glutamatergic neuronal markers, VGLUT1 and 2 and GLS. Fourthly, the potential for simultaneous expression of VGLUT1 and 2 was explored by examination of adjacent serial sections of ICG alternatively stained for VGLUT1 and 2.
Experimental procedures
Adult Sprague-Dawley (SD) rats (n=28, 250–350g) were housed in a 12 h light: 12 h dark cycle and given free access to food and water. All procedures were conducted according to guidelines from the National Institutes of Health (NIH Publications No. 80-23) and were approved by the Oklahoma State University Center for Health Sciences Institutional Animal Care and Use Committee (IACUC #2014-02). All efforts were made to minimize the number of animals used and their suffering.
Surgery
In the retrograde tracing study, SD rats (n=11) were anesthetized with 5% isoflurane and maintained by 3% isoflurane. The depth of anesthesia was tested by the absence of response to pinch of rat hind paw and tail end. To avoid thoracotomy, a trans-diaphragm injection method was chosen. Anesthetized rats were laid supine and the ventral abdominal surgical field was prepared by shaving the hair and cleaning with Providone-iodine. A midline incision was made through the abdominal wall below the xiphoid process exposing the abdominal cavity. The liver was retracted, the xiphoid process was lifted, and the heart was observed through the central tendon of diaphragm. Using a 10µl microinjection syringe, 2–3µl of WGA-HRP (Vector Laboratories, Inc., CA, USA) was injected trans-diaphragmatically into the cardiac ventricular muscle at an angle between 0–15°. A suture knot was placed 1.5–2mm from the tip of injection needle to maintain a consistent depth of injection into the ventricle. Two-three injections were made into the ventricular wall with a total amount of approximately 8 µl of WGA-HRP injected into the ventricular wall. After injections, both the abdominal muscle and skin were sutured. Animals were allowed to recover from the surgery on a warm cloth and were placed in single cages for 72–84 hours. WGA-HRP is transferred to the neuronal cell soma by receptor mediated internalization and active retrograde transport of endosomes (Aschoff and Hollander, 1982, Kobbert et al., 2000, Hoover et al., 2008).
Tissue preparation
Naïve adult rats (n=13) of both sex and rats from the retrograde tracer study (n=11) were intra-peritoneally (i.p.) injected with 2.5% Avertin (3ml) in phosphate buffered saline (PBS) for anesthesia and a subsequent i.p. injection of 1ml xylazine for euthanasia. A thoracotomy was performed to expose the heart and rats were transcardially perfused with 100ml calcium free Tyrodes solution followed by 300ml of 0.2% (w/v) paraformaldehyde, 0.8% (w/v) picric acid solution in 0.2M sodium phosphate buffer. After perfusion, atrial tissue with epicardial fat pad was removed for immunohistochemical evaluation. For absorption control studies, the colon, cornea, skin, sciatic nerve, DRG, trigeminal ganglia (TG), and spinal cord from several rats were removed, also. Tissues were placed in post-fixative for 4 hours at 4°C and stored in 10% sucrose (w/v) at 4°C until immunohistochemical processing. The atrial tissue and other tissues were laid flat in embedding molds, covered with Shandon M-1 embedding matrix (Thermo Scientific, MI, USA) and frozen in a −20°C cryostat chamber. Tissues were cut as 16–20µm frozen sections with a cryostat (Leica CM1850, Leica Biosystems Nussloch GmbH, Germany). Tissues were mounted on gelatin coated Super Frost slides (Fisher Scientific, Pittsburgh, PA, USA) and dried at 38°C on a slide warmer for 90 minutes. After this step, sections were rinsed three times with PBS. To identify ICG neurons in the atrial tissue, some sections were stained tinctorially with a toluidine blue (TB) working solution (10% TB in 1% sodium chloride, pH=2.5) for 90 seconds. Once sections with ICG neurons were identified with light microscopy for TB staining, neighboring slides were selected for immunohistochemistry.
Immunohistochemistry
Atrial tissue sections from naïve rats (n=13) were incubated in mouse anti-peripherin (Millipore/Chemicon, Billerica, MA, USA) and one of the following primary antibodies: rabbit anti-GLS (1:20,000, gift from Dr. N. Curthoys, Colo. St. Univ.), rabbit anti-VGLUT2 (1:2000 HY-19, Sigma, St. Louis, MO), and rabbit anti-VGLUT1 (1:2000,Sigma). Antisera were diluted in PBS with 0.3% (v/v) Triton X-100 (PBS-T) and 2% (w/v) polyvinylpyrolidone, 2% (w/v) bovine serum albumin (PBS-T-PVP-BSA; (Hoffman et al., 2010, Hoffman et al., 2011). Antisera dilutions were determined from previous dilution curve experiments (data not shown). After incubation in primary antibodies for 72hrs at 4°C, sections were rinsed three times with PBS and incubated in secondary antibodies. To detect primary antibodies raised in rabbit (anti-GLS, anti-VGLUT1, anti-VGLUT2), sections were incubated in AlexaFluor 488 goat anti-rabbit IgG (H+L) (1:1500, Invitrogen, Grand Island, NY, USA) in PBS with 0.3% (v/v) Triton X-100 (PBS-T). For concurrent detection of mouse anti-peripherin antiserum, tissue sections also were incubated in AlexaFluor 555 goat anti-mouse IgG (H+L; 1:1500 Invitrogen, Grand Island, NY, USA) in PBS-T. Tissues were incubated in secondary antisera for 2 hours at room temperature followed by rinsing in PBS three times. To identify cell nuclei, tissue sections were incubated in 300nM of 4’,6-diamidino-2-phenylindole (DAPI) in PBS for 10 minutes. Slides were rinsed three times in PBS and coverslips were apposed with ProLong Gold mounting medium (Life Technologies, Grand Island, NY, USA).
In order to evaluate the ventricle injection site or detect retrogradely labeled ICG neurons, tissue sections of ventricle or ICG were incubated in biotinylated goat anti-wheat germ agglutinin (WGA) (1:300,Vector laboratories, Burlingame, CA, USA) in PBS-T-PVP-BSA. To evaluate if ICG retrogradely labeled neurons contained glutamatergic proteins, double immunohistochemistry studies were performed. Rabbit anti-GLS (1:20,000), rabbit anti-VGLUT2 (1:2000) or rabbit anti-VGLUT1 (1:2000) was co-incubated with biotinylated goat anti-WGA in PBS-T-PVP-BSA. Tissues were incubated in primary antisera in 4°C for 72hours and rinsed 3 times with PBS. For detection of rabbit anti-GLS, -VGLUT2 or -VGLUT1, Cy3 conjugated affinipure donkey anti-rabbit IgG (Jackson Immunoresearch, West Grove, USA) was used at 1:2500 dilution in PBS-T for 2 hours in room temperature. After this step, tissues were rinsed 3 times in PBS and processed for detection of WGA-HRP with tyramide signal amplification (TSA kit #22, Invitrogen, Molecular Probes, NY, USA). Tissue sections were incubated in streptavidin-HRP diluted 1:1000 in Tris-Saline with 1% (w/v) BSA for 1 hour at room temperature. Tissue sections were rinsed three times in PBS and placed flat in a humidity chamber. Alexafluor 488 tyramide (1:400; 150µl) in amplification buffer with 0.3% (v/v) H2O2 was applied to the surface of the tissue and incubated for 10 minutes. After rinsing 3× in PBS, DAPI was used for nuclei identification as previously described. After rinsing 3 times in PBS, coverslips were applied to sections with ProLong Gold mounting medium.
In another set of experiments, male Sprague Dawley rats (n=3) were anesthetized and perfused with fixative as described earlier. To evaluate the presence of cholinergic phenotype in ICG neurons and their relationship with glutamatergic ICG neurons, rat atrium tissues were cut (10µm) and co-stained with immunohistochemistry for vesicular acetylcholine transporter (VAChT; 1:2000; goat anti-VAChT [N-19; sc-7717]; Santa Cruz, Dallas, TX, USA) and rabbit anti-VGLUT1 (1:2000), -VGLUT2 (1:2000) and -GLS (1:20,000). The antiserum concentration for goat anti-VAChT was determined from a dilution curve experiment (data not shown). After incubation in primary antibodies for 72hrs at 4°C, sections were rinsed three times with PBS and incubated in secondary antisera. The secondary antisera were AlexaFluor 555 donkey anti-goat IgG (H+L; 1:1500, Invitrogen) in (PBS-T) to detect goat anti-VAChT and AlexaFluor 488 donkey anti-rabbit IgG (H+L; 1:1500, Invitrogen) to detect primary antisera raised in rabbit (VGLUT1 & 2, GLS). To identify cell nuclei, tissue sections were incubated in 300nM of DAPI in PBS for 10 minutes. Slides were rinsed three times in PBS and coverslips were apposed with ProLong Gold mounting medium.
To investigate VGLUT1 and VGLUT2 protein co-expression in neurons of rat ICG, rat atrium tissues (n=3) were cut (10µm) and sequential tissue sections were stained alternatively for rabbit anti-VGLUT1 (1:2000) or rabbit-VGLUT2 (1:2000). Tissues were incubated in secondary antisera (AlexaFluor 488 donkey anti-rabbit IgG (H+L; 1:1500)) for 2 hours at room temperature followed by rinsing in PBS three times. Tissue sections also were incubated in 300nM of DAPI in PBS for 10 minutes. Slides were rinsed three times in PBS and coverslips were apposed with ProLong Gold mounting medium.
Antibody controls: To examine the specificity of the antisera, three controls were performed. First, omission of individual primary antisera served as negative controls. Secondly, primary antisera were incubated overnight with their respective purified antigens (e.g., 2% VGLUT2 peptide solution combined with 1:1000 rabbit anti-VGLUT2 antibody in PBS-T-PVP-BSA). The pre-absorbed antiserum was applied to tissue (ICG, colon, skin, cornea, sciatic nerve, DRG, TG, spinal cord) for 72 hr followed by secondary antiserum incubation as described above. Thirdly, to ensure that combinations of primary and secondary antisera were free from cross reactivity, we processed primary antisera from one species with the secondary antiserum raised for the different species (e.g., rabbit anti-VGLUT2 with Alexaflour 555 goat anti-mouse IgG).
Image analysis
Images were acquired with a 40× objective on a BX51 epifluorescence microscope (Olympus; Center Vally, PA, USA) equipped with a SPOT RT740 camera (Diagnostic instruments; Sterling Heights, MI, USA). The image acquisition method is similar to previous studies from our laboratory (Hoffman et al., 2010, Hoffman et al., 2011). Briefly, an appropriate exposure time was determined based on the criteria that the dimmest region of the cell could be discerned visually for image analysis tracing, but the pixels for the brightest regions were not over saturated (>255 pixels). Five visual fields were captured for each tissue section with three different channels: FITC (Green), TRITC (Red) and DAPI (Blue) and at least three slides were used from each animal. The individual ICG tissue sections were chosen at a minimum of 80µm apart in order to avoid cell overlap during data analysis. A minimum of 45 images per animal were obtained for image analysis. Neuron profiles with clearly visible nuclei and without touching the edge of the image were traced with a Cintiq 21UX interactive pen display (Wacom; Kita Saitama-Gun, Saitama, Japan) using the freehand selection tool in Image J software (National Institutes of Health, Bethesda, MD, USA). For single ICG neurons, the cytoplasmic profile was traced by excluding the cell nuclei from the cell region of interest (ROI). The cell area in µm2 for each cell and the mean gray value for each cytoplasmic profile were measured and transferred to a spreadsheet. A minimum of 100 neurons per animal for each marker used in each double/triple labeled combination was evaluated and recorded.
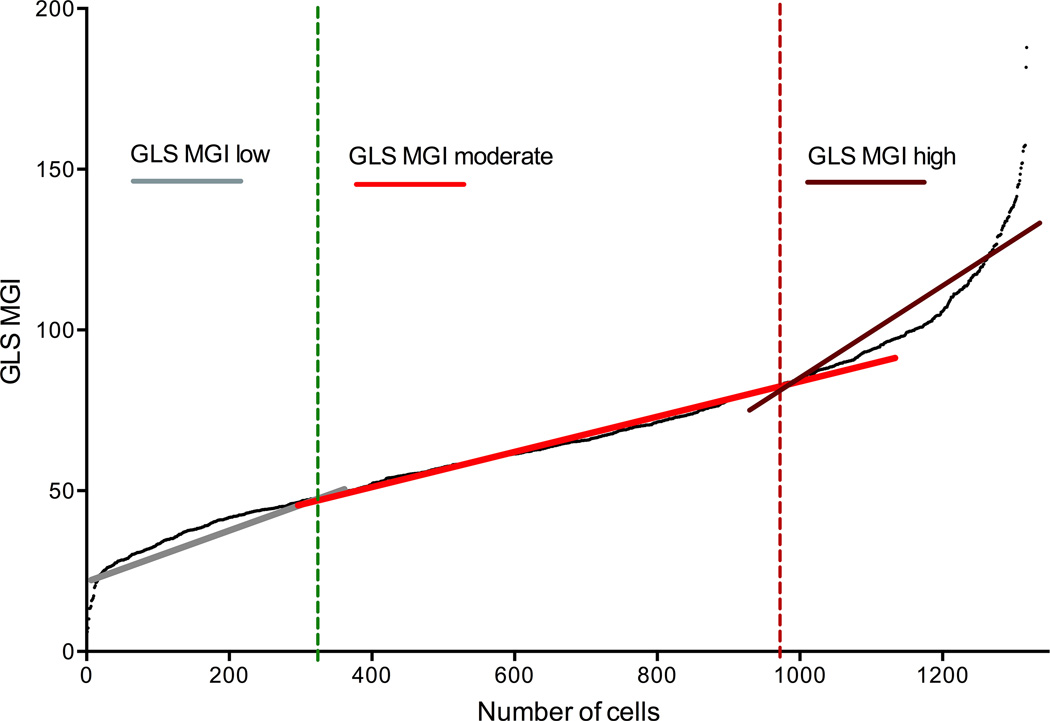
For GLS, VGLUT1, and VGLUT2, all cells were ranked by their mean gray intensity (MGI) and an immunoreactivity curve was developed using the cell ranking for the x-axis and MGI for the y-axis (GLS example in Figure 2). The curve showed three broad categories of immunoreactivity for ICG neurons. Using linear regression for each part of the curve and the intercepts of the lines, neurons ranked in the first 25% of the population were described by the first regression line, neurons in the 25–75% ranking by the second regression line, and neurons ranked in the 75–100% portion by the third regression line (Figure 2). This type of immunoreactive profile occurred for each of the three antisera (VGLUT 1 & 2 curves not shown).
Figure 2.
Mean gray intensity sorting classification. This graph illustrates how low, moderate, high immunoreactive cells were determined for the study. In this example, all GLS-immunoreactive ICG neurons were ranked by their mean gray intensity (MGI) and a curve (black line) was developed using the cell ranking for the x-axis and MGI for the y-axis. The curve shows three categories of GLS-immunoreactivity for ICG neurons. Linear regression was performed for each part of the curve (gray, red, and dark red lines). The intercepts of the lines were used for determining the cut-off for each category (green and red dotted lines). Neurons within the first 25% of the population were categorized as having low immunoreactivity. Neurons in the 25–75% ranking and in the 75–100% ranking were categorized as having moderate or high immunoreactivity, respectively.
To evaluate VGLUT1 and VGLUT2 immunoreactivity in sequential tissue sections, neighboring atrium tissue sections (10 µm thickness) were photographed with a 40× objective. The Mosaic J plug-in of Image J software was used to re-create a large-scale transverse section of the atrial ICG plexus (5 pictures for each mosaic photo). VGLUT1 and VGLUT2 stained photos were aligned side-by-side, individual neurons present in both sections were identified and evaluated for immuno-intensity for VGLUT1 and VGLUT2.
Statistics
Prism version 5.01 (Graph Pad Software, Inc.; LaJolla, CA, USA) was used to perform statistical tests and construct graphs. Frequency distribution histograms of the cell area were plotted for all the ICG cells measured. The histogram distribution of cell sizes for each antiserum was tested for normality with the D’Agostino and Pearson omnibus normality test, Shapiro-Wilk normality test and Kolnogorov-Smirnov test normality test. There was deviation from normality for the data sets, therefore both mean and median values were reported. The frequency distribution of the immunohistochemically labeled ICG neurons was fitted by conducting a nonlinear Gaussian distribution test. To compare the mean cell size of WGA-HRP labeled neurons to unlabeled neurons and the ICG neurons based on GLS, VGLUT1 and VGLUT2 immunoreactivity (ir), one way analysis of variance (ANOVA) tests followed by post hoc Tukey tests were conducted. P values less than 0.05 were considered significant. The specific statistical tests used are indicated in the figure legends.
Results
Glutamatergic neurons in rat intrinsic cardiac ganglia
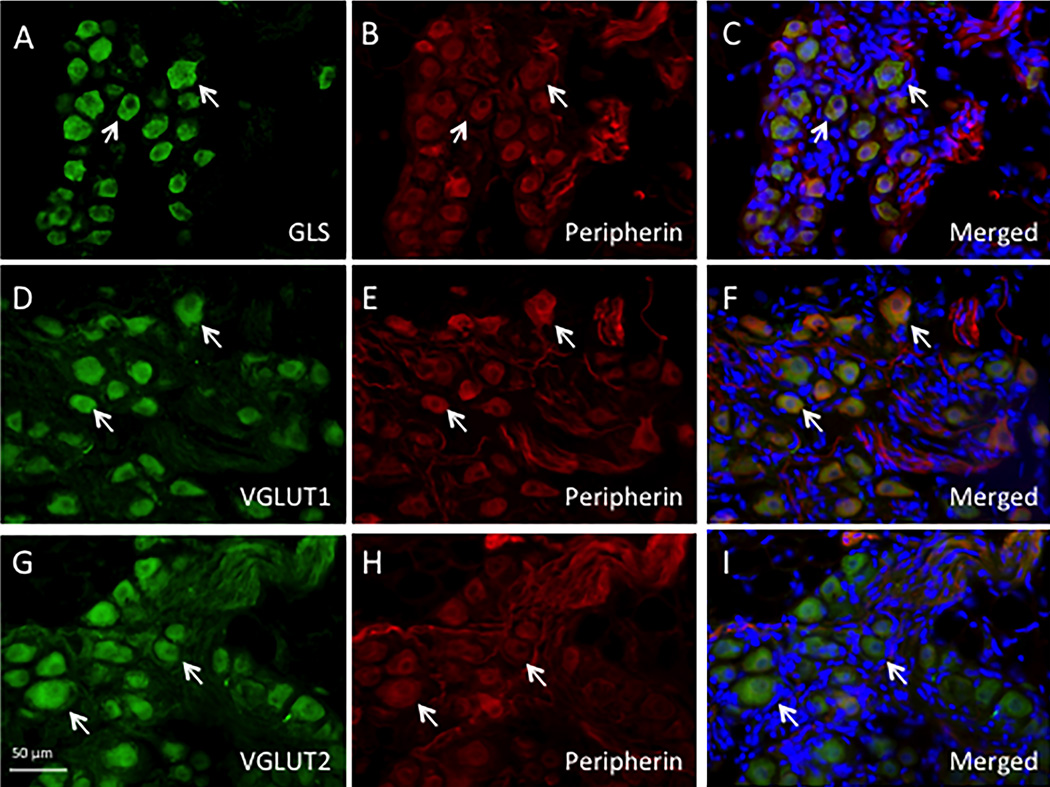
Double immunolabeling of rabbit anti-GLS/-VGLUT1/-VGLUT2 with mouse anti-peripherin and DAPI nuclear staining showed distinct ICG neuronal cell profiles (Figure 1C,F,I). In addition, the ICG contained large bundles of nerve fibers (predominantly peripherin-immunoreactive) coursing through the ganglia (Figure 1B,E,H).
Figure 1.
(A–I): Immunohistochemistry study for GLS, VGLUT1 and VGLUT2 in rat intrinsic cardiac ganglia. A–C: GLS-ir in ICG neurons (arrows). Punctate GLS-ir was present throughout the neuronal cytoplasm, but GLS-ir was low in the peripherin identified nerve fibers (B,C). D–F: VGLUT1-ir in ICG neurons (arrows) had an even appearance throughout the cytoplasm. G–I: VGLUT2-ir in ICG neurons (arrows) displayed a uniform cytoplasmic staining. B,E,H: Peripherin-ir was used to identify ICG neurons within the atrial ganglia. A large number of peripherin-IR nerve fibers coursed through ICG ganglia. C,F,I: Triple labeling including staining with DAPI for individual cell nuclei (blue) in merged pictures.
GLS-, VGLUT1-, and VGLUT2-IR neurons were classified as having low, moderate, or high immunoreactivity (ir) based on their mean gray intensity (MGI) ranking (Figure 2). Low immunoreactive (IR) neurons occurred in the first 25% (<25%) of ranking, moderate IR neurons were in the 25–75% ranking, and high IR neurons were in 75–100% ranking (Figure 2). Blinded observer ratings confirmed that neurons in the <25% category were low- to non-IR and were excluded from further evaluation.
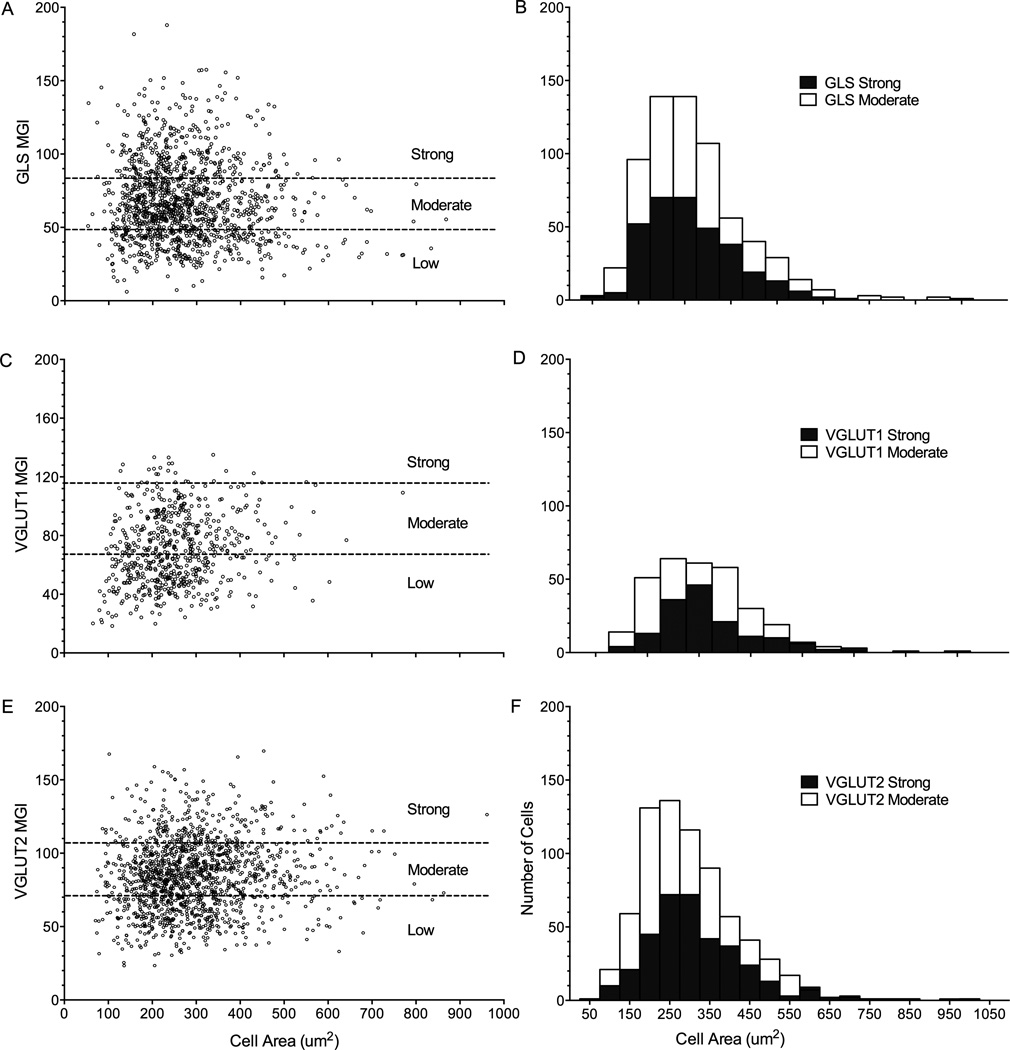
GLS-ir was present in many rat ICG neurons as puncta found throughout the cytoplasm (Figure 1A,C; Hoffman et al., 2010; Miller et al., 2012; Crosby et al., 2015; Hoffman et al., 2016). Among 1318 ICG neurons analyzed (n=5 rats), the cell soma area ranged from 53.0 to 867.9µm2 (Figure 3A). ICG neurons were classified into three groups based on GLS-ir (MGI) (Figure 2, 3A). Neurons with low GLS-ir ranged from 6.2–48.1 MGI (lowest 25%), whereas neurons with moderate GLS-ir ranged from 48.2–83.9 MGI (25–75%) and neurons with high GLS-ir ranged from 83.9–187.8 MGI (highest 25%; Figure 3A). Only GLS-IR neurons with moderate to high immunostaining were used for further histogram distribution analysis (Figure 3B). The cell area for strong GLS-IR neurons ranged from 54.0–623.7µm2 with a mean of 263.4 µm2 and median of 242.4µm2 (Figure 3B). The cell area for moderate GLS-IR neurons ranged from 53.0–868.0µm2 with a mean of 268.3µm2 and median of 247.9µm2 (Figure 3B).
Figure 3.
(A–F): Distribution of glutamatergic neurons in rat intrinsic cardiac ganglia. A: Glutaminase-immunoreactive (IR) neuronal cells were plotted by cell area vs mean gray intensity (MGI) and classified into groups of low, moderate, or strong immunoreactivity (ir). Neurons with moderate- and strong-ir were chosen for further analysis. B: Frequency distribution of medium and strong GLS-IR neurons. The mean cell area of moderate and strong GLS-IR neurons was 268.3and 263.4µm2, the median cell area of moderate and strong GLS-IR neurons was 247.9 and 242.4µm2, respectively. C: VGLUT1-IR neurons were plotted by cell area vs MGI. D: Frequency distribution of VGLUT1-IR neurons. The mean cell area of moderate and strong VGLUT1-IR neurons was 253.7 and 272.5µm2, the median cell area of moderate and strong VGLUT1-IR neurons was 241.2 and 253.0µm2, respectively. E: VGLUT2-IR neuronal cells were plotted by cell area vs MGI. F: Frequency distribution of VGLUT2-IR neurons. The mean cell area of moderate and strong VGLUT2-IR neurons was 297.8 and 314.6µm2, the median cell area of moderate and strong VGLUT2-IR neurons was 279.9 and 295.2µm2, respectively.
VGLUT1-ir also was present in many rat ICG neurons (Figure 1D,F). In peripherin labeled ICG neurons (Figure 1E), VGLUT1-ir appeared as bright cytoplasmic staining (Figure 1D). Nerve fibers passing through the ICG contained moderate VGLUT1-ir compared to VGLUT1-IR neuronal cell bodies (Figure 1D,F). Among 618 VGLUT1-IR cells (n=3 rats) evaluated, the cell area ranged from 65.3 to 770.6µm2 (Figure 3C). VGLUT1-IR neurons with moderate ir (25–75%, 51.1–87.2 MGI) and high ir (highest 25%, 87.2–135.0 MGI) were used for subsequent histogram distribution analysis (Figure 3D). Neurons with high VGLUT1-ir ranged from 114.9–770.6µm2 with a mean of 272.5µm2 and a median of 253.0µm2 (Figure 3D). Neurons with moderate VGLUT1-ir ranged from 96.3–642.3µm2 with a mean of 253.7µm2 and a median of 241.2µm2 (Figure 3D).
VGLUT2-ir occurred in rat ICG neurons as a smooth cytoplasmic staining pattern (Figure 1G,I; Crosby et al., 2015). Large nerve bundles penetrating through the ICG also had VGLUT2-ir (Figure 1G–I). Among the 1424 VGLUT2-IR (n=5 rats) cells, moderate IR neurons (25–75%, 68.4–100.2 MGI) and high IR neurons (highest 25%, 100.2–169.6 MGI) were used for subsequent histogram frequency analysis (Figure 3E,F). Neurons with moderate VGLUT2-ir ranged 74.1 to 1004.8µm2 with a mean of 297.8µm2 and a median of 279.9µm2 (Figure 3F). Strong VGLUT2-IR neurons ranged 86.7–961.6µm2 with a mean of 314.6µm2 and a median of 295.2µm2 (Figure 3F).
According to previous studies, classifications of ICG neurons can be based on their size and immunostaining (Armour, 1999, Horackova et al., 2000, Richardson et al., 2003, Rysevaite et al., 2011). Generally, neurons with a radius <10µm are considered as small neurons (<314µm2) and with radius >10µm are considered as large neurons (>314µm2). When these criteria are applied to our results, 76.9% of GLS-IR neurons were small and 69.8% were large; 73.4% of VGLUT1-IR neurons were small and 81.4% were large; 77.4% of VGLUT2-IR neurons were small and 72.6% were large.
Retrograde tracing studies
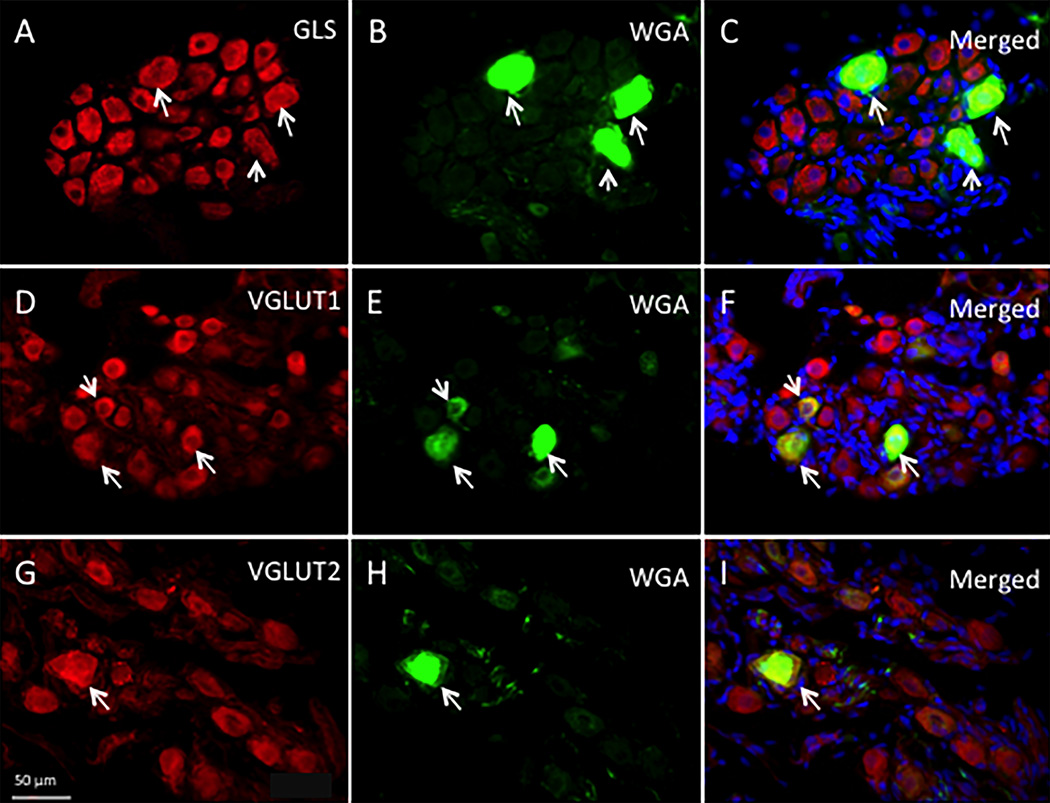
In WGA-HRP retrograde tracing studies, ICG neurons were identified by WGA-HRP transport from the ventricular wall to the atrial ICG (Figure 4B,E,H). Retrogradely labeled neurons also were immunostained for GLS, VGLUT1, or VGLUT2 (Figure 3A–I). In order to maintain minimal changes between control rats and retrograde labeling rats, we used animals in similar age with close body weight for both groups. The experimental processes of tissue gathering and immunohistochemistry were kept standard for each group. To evaluate if the retro-diaphragm surgery or WGA-HRP transport caused changes in GLS, VGLUT1 and VGLUT2-IR populations, we compared the Gaussian distribution from the retro-diaphragm surgery (Figures 5–7B) and control animal groups (Figure 3B,D,F). The groups showed similar cell profile distribution curves (positive skewness) without distinct cell population changes in each group (Figure 3B,D,F vs. Figures 5–7B).
Figure 4.
(A–I): Retrograde labeling and immunohistochemistry for rat ICG neurons (arrows). A–C: GLS-IR neurons in rat ICG. D–F: VGLUT1-IR neurons in rat ICG (red).G–I: VGLUT2-IR neurons in rat ICG (red). B, E, H: WGA-HRP retrogradely labeled cells (Green); C, F, I: Merged pictures of GLS, VGLUT1, or VGLUT2 staining and WGA-HRP staining. DAPI staining (blue) also was performed to show individual cell nuclei.
Figure 5.
(A–C): GLS-immunoreactive (IR) neurons in the retrograde labeling study. A: GLS-IR neurons from the WGA-HRP retrograde labeling study were plotted by area vs. MGI. Open circles indicate non-retrogradely labeled neurons and filled circles indicate retrogradely labeled neurons. Neurons were classified into groups of low, moderate, and strong GLS-immunoreactivity (ir). Neurons with moderate and strong GLS-ir were chosen for subsequent analysis. B: Histogram distribution of WGA-HRP retrogradely labeled neurons and non-retrogradely labeled neurons. The mean neuronal profile of retrogradely labeled neurons was 400.7µm2 and the mean neuronal profile of non-retrogradely labeled neurons was 282.6µm2. C: One way ANOVA compared the mean cell areas of retrogradely labeled neurons (Mean ± SEM: 387.2± 21.7µm2), non-retrogradely labeled neurons (Mean ± SEM: 276.4 ±17.2µm2) and total neurons (Mean ± SEM: 284.8±16.5µm2). Retrogradely labeled neurons were statistically larger than the total population and the non-retrogradely labeled neurons (P value = 0.004).
Figure 7.
(A–D): VGLUT2-IR neurons in the retrograde labeling study. A: VGLUT2-IR neurons from the WGA-HRP retrograde labeling study were plotted by area vs. MGI. Open hexagons indicate non-retrogradely labeled neurons and filled hexagons indicate retrogradely labeled neurons. Neurons were classified into groups of low, moderate, and strong VGLUT2-ir. Neurons with moderate and strong immunoreactivity were chosen for subsequent analysis. B: Histogram distribution of WGA-HRP retrogradely labeled neurons and non-retrogradely labeled neurons. The mean neuronal profile of retrogradely labeled neurons was 399.25 µm2 and the mean neuronal profile of non-retrogradely labeled neurons was 304.03µm2. C: One way ANOVA compared the mean cell area of retrogradely labeled neurons (Mean± SEM: 396.4 ± 45.7µm2), non-retrogradely labeled neurons (Mean± SEM: 298.9 ± 16.8µm2) and total neurons (Mean± SEM: 307.2 ±18.4 µm2). Retrogradely labeled neurons show a trend of increase in size, but the mean cell sizes were distributed widely in the retrogradely labeled group, so statistical significance was not achieved. (P value= 0.113). D: The average sizes of retrogradely labeled, GLS-, VGLUT2, and VGLUT1-IR neurons were compared with one way ANOVA. Although the VGLUT1-IR group appeared larger, there was no significance difference in the groups (P value = 0.14).
For GLS, 548 neurons were analyzed (n=4 rats). The GLS-IR cells ranged 79.2–930.3µm2 (Figure 5A). Further analysis used GLS-IR neurons with moderate (62.8–100.7 MGI) and high (100.8–199.0 MGI) immunostaining (Figure 5A). Among these GLS-IR cells (n=411), 27 neurons (7%) were retrogradely labeled with WGA-HRP. GLS-IR, retrogradely labeled neurons were significantly larger (mean 363.8µm2, median 400.7µm2) than GLS-IR, retrogradely unlabeled neurons (mean 282.6µm2, median 260.6µm2; p<0.01; Figure 5B,C).
For VGLUT1 (n=3 rats), 696 neurons were identified, ranging 91.0–869.0µm2 (Figure 6A). Moderate VGLUT1-IR neurons (67.2–102.3 MGI) and high VGLUT1-IR neurons (102.4–174.6 MGI) were used for analysis (Figure 6A). Among VGLUT1-IR cells (n=554), 35 neurons (6%) were identified as WGA-HRP labeled. The size of VGLUT1-IR, retrogradely labeled neurons (mean 493.0µm2, median 464.9µm2) was larger than VGLUT1-IR, retrogradely unlabeled neurons (mean 314.7µm2, median 290.7µm2; p<0.01; Figure 6B,C).
Figure 6.
(A–C): VGLUT1-IR neurons in the retrograde labeling study. A: VGLUT1-IR neurons from the WGA-HRP retrograde labeling study were plotted by area vs. MGI. Open diamonds indicate non-retrogradely labeled neurons and filled diamonds indicate retrogradely labeled neurons. Neurons were classified into groups of low, moderate, and strong VGLUT1-immunoreactivity (ir). Neurons with moderate and strong VGLUT1-ir were chosen for subsequent analysis. B: Histogram distribution of WGA-HRP retrogradely labeled neurons and non-retrogradely labeled neurons. The mean neuronal profile of retrogradely labeled neurons was 493.0 µm2 and the mean neuronal profile of non-retrogradely labeled neurons was 314.7 µm2. C: One way ANOVA test compared the mean cell areas of retrogradely labeled neurons (Mean ± SEM: 476.9± 21.6µm2), non-retrogradely labeled neurons (Mean ± SEM: 316.0 ± 25.6µm2) and total neurons (Mean ± SEM: 326.4 ± 29.7 µm2). Retrogradely labeled neurons were statistically larger than the total population and the non-retrogradely labeled neurons (P value= 0.008).
For VGLUT2 (n=3 rats), a total 441 neurons were identified, ranging 93.1–1008.8µm2 in cell area (Figure 7A). Moderate VGLUT2-IR neurons (103.1–150.4 MGI) and high VGLUT2-IR neurons (150.4–243.1 MGI) were used for analysis (Figure 7A). Among VGLUT2-IR cells (n=331), 26 neurons (8%) were identified as WGA-HRP labeled. The cell size of VGLUT2-IR, retrogradely labeled neurons (mean 399.3µm2, median 378.1µm2) appeared larger than VGLUT2 retrogradely unlabeled neurons (mean 304.0µm2, median 282.8µm2, Figure 7B,C). No statistical difference, however, was determined (P = 0.113) due to the large variability in the VGLUT2-IR, retrogradely labeled neuronal group (S.D. = 79.1, SEM = 45.7). Comparison of the average size of retrogradely labeled, GLS-, VGLUT1-IR, and VGLUT2-IR groups did not show significant differences among the populations, although the VGLUT1-IR group appeared larger (Figure 7D).
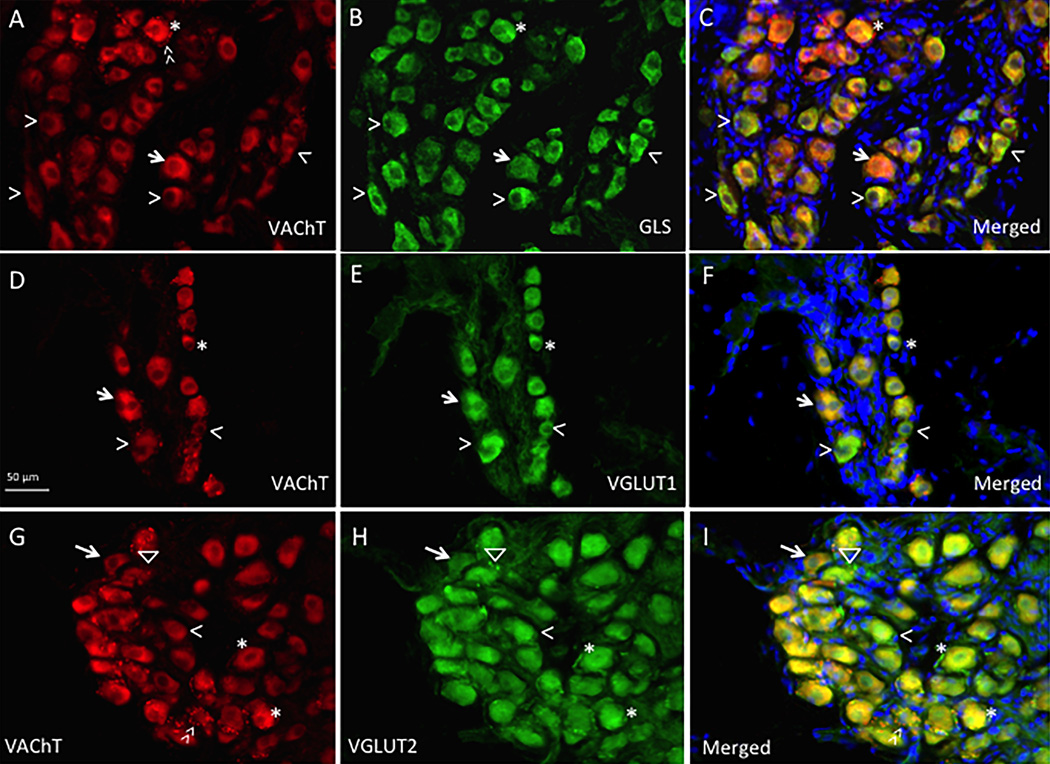
In the VAChT and glutamatergic marker double-labeling studies, many GLS-, VGLUT1- & 2-IR neurons also contained VAChT-ir (Figure 8A–I). Based on the analysis of immunointensity for each protein, a large portion of population of ICG neurons contained medium/strong ir for both VAChT and GLS/VGLUT1/VGLUT2 (Figure 8). Some moderate/strong VAChT-IR neurons were low in GLS/VGLUT1/VGLUT2-ir (Figure 8), whereas some moderate/strong GLS/VGLUT1/VGLUT2-IR neurons contained low VAChT-ir (Figure 8). Among moderate and strong GLS-IR neurons, 81% (408/499) of GLS neurons also were moderate to strong VAChT-IR. Among moderate and strong VGLUT1-IR neurons, 85.5% (431/504) of VGLUT1 neurons also had moderate to strong VAChT-ir. Among moderate and strong VGLUT2-IR neurons, 88.9% (461/518) of neurons also were moderate to strong VAChT-IR.
Figure 8.
(A–I): Double-labeling immunohistochemistry study for VAChT and glutamatergic neurons in rat intrinsic cardiac ganglia. A–C: VAChT and GLS-immunoreactivity (ir) in ICG neurons. D–F: VAChT and VGLUT1-ir in ICG neurons. G–I: VAChT and VGLUT2-ir in ICG neurons. A–H: VAChT-ir (A,D,G) was present in many ICG neurons that were GLS/VGLUT1/VGLUT2-immunoreactive (IR; B,E,H). A population of ICG neurons contained moderate to strong ir for both VAChT and GLS/VGLUT1/VGLUT2 (asterisks); some moderate to strong VAChT-IR neurons were low in GLS/VGLUT1/VGLUT2-ir (arrows); and some moderate/strong GLS/VGLUT1/VGLUT2-IR neurons contain low VAChT-ir (arrowheads). C,F,I: Merged images for double labeling including DAPI nuclear staining. Neurons with strong VAChT and GLS/VGLUT1/VGLUT2-ir appear an orange color (asterisks); neurons with moderate/strong VAChT-ir and low GLS/VGLUT1/VGLUT2-ir appear a more reddish color (arrows); neurons with low VAChT and strong GLS/VGLUT1/VGLUT2-ir appear a greenish color (arrowheads). VAChT-IR varicose nerve fibers were observed to surround ICG neurons (bright red fiber in A,G - double arrowhead) and VGLUT2-IR varicose nerve fibers also were observed to surround ICG neurons (bright green fiber in H,I - triangle).
VGLUT1 and VGLUT2-ir in sequential atrial ICG sections showed ICG neurons with both VGLUT1 and VGLUT2-ir (Figure 9A–D). Differences in the amount of VGLUT1- and VGLUT2-ir in the same ICG neurons were noticed (Figure 9E–H). Some strong VGLUT1-IR neurons contained moderate VGLUT2-ir (Figure 9E,F), whereas some moderate VGLUT1-IR neurons had very low VGLUT2-ir (Figure 9G,H).
Figure 9.
(A–F): VGLUT1 and VGLUT2 colocalization in rat intrinsic cardiac ganglia. Alternate staining of VGLUT1 (A,C) and VGLUT2 (B,D) in sequential tissue sections containing rat ICG atrium neurons (A–D). E,F: Some strong VGLUT1-IR neurons (E) had moderate VGLUT2-ir (F). G,H: Some moderate VGLUT1-IR neurons (G) contained low VGLUT2-ir (H).
Absorption controls and retrograde label injection site
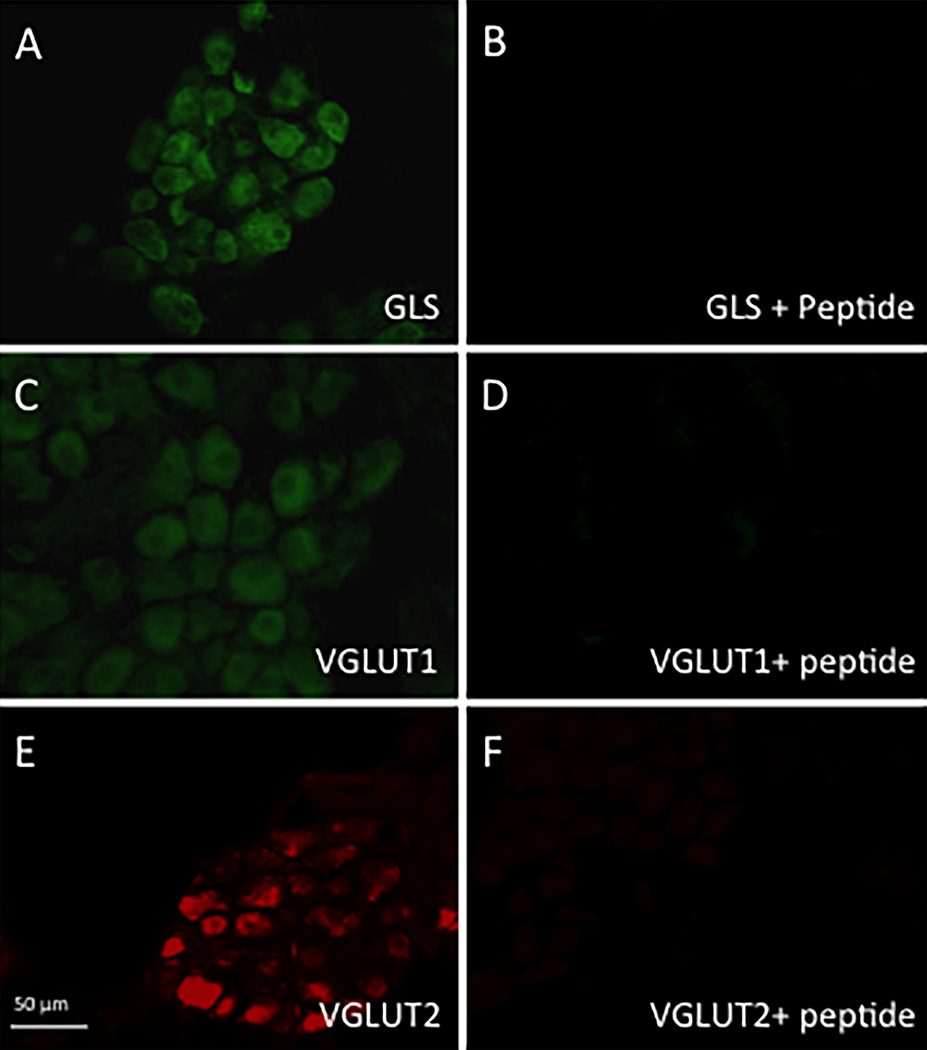
When primary antisera were omitted and tissues were only incubated with secondary antibodies, no immunostaining was observed (data not shown). When primary antisera were preabsorbed with their respective antigens, the ICG was void of immunostaining (Figure 10A–F). The colon, skin, cornea, sciatic nerve, DRG, TG, spinal cord tissues also did not have immunostaining with preabsorbed primary antisera (data not shown). The use of a mismatched secondary antibody showed no cross reaction between primary and secondary antibodies (data not shown). Ventricle tissue sections at the retrograde label injection site showed an area around the injection track that was labeled for WGA-HRP (Figure 11).
Figure 10.
(A–F): Antiserum absorption controls. A: GLS-ir in rat ICG neurons with secondary Alexaflour488 goat anti-rabbit IgG. B: Rabbit anti-GLS pre-absorbed with GLS peptide eliminated GLS-IR. C: VGLUT1-ir in rat ICG neurons with secondary Alexaflour 488 goat anti-rabbit IgG. D: Rabbit anti-VGLUT1 pre-absorbed with VGLUT1 peptide dramatically decreased VGLUT1-ir. E: VGLUT2-ir in rat ICG neurons with secondary Cy3 donkey anti-rabbit IgG. F: Rabbit anti-VGLUT2 pre-absorbed with VGLUT2 peptide abolished VGLUT2-ir.
Figure 11.
Ventricular injection site. Rat ventricle tissue stained for Goat anti-WGA-HRP to detect the retrograde tracer injection site. The black area outlined by arrowheads illustrates the needle tract from the microinjection syringe. The area around the injection site (arrows) is green indicating the residue of the WGA-HRP retrograde tracer.
Discussion
In the present study, we evaluated the presence of glutamatergic neurons in rat intrinsic cardiac ganglia by utilizing immunohistochemistry (IHC) and retrograde tracing methods. Our results demonstrated: 1) Rat ICG neurons contain GLS-ir, the synthetic enzyme for glutamate production; 2) Rat ICG neurons contain VGLUT1&2-ir, transporters for glutamate into synaptic vesicles; 3) Within the ICG, GLS-, VGLUT1- and VGLUT2-IR cells had similar cellular profiles with mean and median cell sizes ranging in area 250–315µm2 and 241–295µm2, respectively; 4) Some atrial ICG neurons send axons to the ventricular wall (WGA-HRP retrograde labeling) and express GLS, VGLUT1 & 2; 5) Retrogradely labeled, glutamatergic ICG neurons were larger (mean area 399–492µm2, median 378–465µm2) than other glutamatergic ICG neurons (mean area 282–315µm2, median 260–290µm2); 6) A large population (81–89%) of glutamatergic ICG neurons were also cholinergic with VAChT-ir; 7) Some ICG neurons expressed both VGLUT1 and VGLUT2, but the level of expression for the two transporters varied among neurons.
Glutamate as a neurotransmitter in ICG neurons
Glutamate is the major excitatory neurotransmitter in the vertebrate central nervous system (Fremeau et al., 2004, Wojcik et al., 2004) and is used by primary sensory neurons in TG (Yang et al., 2014) and DRG (Brumovsky et al., 2007, Miller et al., 2011) of the peripheral nervous system. In primary sensory neurons in DRG, neurotransmitter glutamate production utilizing the glutamate-glutamine cycle is important for sensory function (Miller et al., 1993, Miller et al., 2002, Miller et al., 2012). Phosphate-activated GLS, regulated by calcium (Ca2+) and inorganic phosphate (Pi), is the synthetic enzyme for conversion of glutamine into glutamate for use as a neurotransmitter (Erecinska et al., 1990, Miller et al., 2011). In the current study, a large population of rat ICG neurons contained GLS indicating that many ICG neurons produce glutamate. Glutamatergic neurons use vesicular glutamate transporters (VGLUTs) 1–3 for packaging neurotransmitter glutamate into synaptic vesicles and VGLUTs are considered reliable markers for glutamatergic neurons (Takamori et al., 2000, 2001, Malet et al., 2013). Among them, VGLUT1 and VGLUT2 have been more intensively studied due to the availability of reliable specific antibodies. Both VGLUT1 & 2 are present in somatic and visceral DRG sensory neurons (Hwang et al., 2004, Brumovsky et al., 2007, Miller et al., 2011, Yang et al., 2014), in enteric neurons (Brumovsky et al., 2011), and in vagal afferent neurons innervating the gastrointestinal tract and heart (Lawrence and Jarrott, 1994, Lawrence, 1995, Berthoud et al., 1997, Corbett et al., 2005, Raab and Neuhuber, 2007). In the current study, the discovery of many neurons expressing GLS, VGLUT1, and VGLUT2 provides novel information regarding the presence of glutamatergic neurons in rat cardiac intrinsic ganglia.
GLS, VGLUT1, and VGLUT2 immunoreactivity occurred in many neurons of the rat ICG. The antisera used for GLS, VGLUT1, and VGLUT2 have been employed previously in our laboratory for rat DRG neurons (Miller et al., 2012, Crosby H., 2015, Bolt, 2016, Hoffman et al., 2016) and pre-absorption control studies in ICG and other rat tissues (colon, skin, cornea, sciatic nerve, DRG, TG, spinal cord) support the specificity of the three antibodies. Furthermore, quantitative image analysis allowed us to categorize immunoreactive neurons into moderate and high classes and exclude neurons that were non-immunoreactive. Classification of moderate and high immunoreactivity also may provide information regarding the relative protein expression level in individual ICG neurons.
In the rat peripheral nervous system, VGLUT1 and VGLUT2 expression patterns vary according to the neuronal circuitry. For instance, VGLUT2 is the major vesicular glutamate transporter for vagal afferent neurons to the stomach (Corbett et al., 2005), but VGLUT1 is utilized by vagal afferents innervating the heart (Corbett et al., 2005). In the rat esophagus, VGLUT2 is used by vagal afferents (Raab and Neuhuber, 2003), while VGLUT1 is more intensively expressed in myenteric ganglia (Ewald et al., 2006). In the current study, VGLUT1 and VGLUT2 occurred in many rat intrinsic cardiac ganglion neurons and neurons expressing VGLUT1 vs. VGLUT2 could not be distinguished based on their size in either naïve rat ICG neurons or retrogradely labeled neurons. This may be due to the large amount of co-expression of VGLUT1 and 2 in ICG neurons as determined from alternatively stained, sequential tissue sections. This is unique for the ICG plexus compared to other peripheral neurons such as DRG sensory neurons (Oliveira et al., 2003, Brumovsky et al., 2007, Brumovsky et al., 2011). In DRG, neurons with large area profiles express VGLUT1 and are mechano-sensitive (proprioceptive), while many small to medium sized neurons express VGLUT2 and are nociceptive (Landry et al., 2004, Brumovsky et al., 2007, Lagerstrom et al., 2011, Yang et al., 2014). VGLUT2 is expressed more broadly in DRG neurons including small, medium and large neurons, while VGLUT1 is observed in discrete subpopulations of mostly large and medium visceral and non-visceral neurons (Malet and Brumovsky, 2015). In rat trigeminal ganglion neurons, colocalization of VGLUT1 and VGLUT2 occurred in 75% of neuronal cell bodies and axon terminals projecting to superficial layers of medullary dorsal horn also showed co-localization of VGLUTs (Li et al., 2003). Although serial sections immunostained for VGLUT1 & 2 showed that many ICG neurons express both glutamate vesicular transporters, the level of VGLUT1 and VGLUT2 expression differed in individual ICG neurons. Previously, co-localization of VGLUT1 and VGLUT2 in vagal mechanical terminals in esophagus tunica muscularis has been reported with a difference in immunointensity of VGLUTs in individual nerve terminals (Ewald et al., 2006). While a differential expression of VGLUT1 vs. VGLUT2 in individual ICG neurons was observed, further detailed studies with peptide or sodium channel co-staining for determining ICG subpopulations or mRNA in situ hybridization for VGLUT expression levels may help in understanding this phenomenon.
ICG neurons previously have been considered as cholinergic in nature (Pauza et al., 2002, Yasuhara et al., 2007) with possible neuronal phenotype flexibility during neonatal development (Horackova et al., 2000). VAChT is a reliable marker for cholinergic neurons (Schafer et al., 1998) and, in current study, the majority (81–89%) of rat ICG neurons that contain GLS/VGLUT1/VGLUT2 also are cholinergic (VAChT), but not all ICG neurons. The presence of both acetylcholine and glutamate in the same neurons has been demonstrated previously in spinal cord motor neurons (Meister et al., 1993, Herzog et al., 2004) and enteric ganglion neurons (Liu et al., 1997, Tong et al., 2001, Ewald et al., 2006). Some researchers suggested glutamate release from motor neurons has a modulatory function in peripheral acetylcholine release (Hochman and Schmidt, 1998, Kiehn et al., 2000, Landry et al., 2004). Some have suggested glutamate as co-transmitter with acetylcholine in somato-, visceral motor neurons (Senba et al., 1991). In the spinal cord ventral horn, Herzog et al, 2004 and Landry et al, 2004 demonstrated presence of VGLUT1 and VGLUT2 immunoreactivity in motor neuron axons distinct from cholinergic axon terminals from same motor neurons, indicating the possibility of independent glutamatergic and cholinergic sorting into different neuronal branches. A similar mechanism has been proposed for the enteric nervous system (Tong et al., 2001, Ewald et al., 2006). The simultaneous release of acetylcholine and glutamate or the differential targeting of selective neurotransmitter release requires further investigation in the ICG. Detailed analysis of protein expression levels of GLS/VGLUT1/VGLUT2 vs. VAChT in individual ICG neurons during development or pathophysiological conditions may provide more information regarding the glutamatergic and cholinergic phenotypes of ICG neurons.
The injection of retrograde tracer WGA-HRP into the ventricular wall provided evidence for glutamatergic neurons projecting to ventricular walls. These neurons are available anatomically for sensing environmental changes from cardiac ventricles. Previous studies indicated that epicardium mechanical and chemical (adenosine, substance P, protons, etc.) stimulation of the ventricular wall induces neuronal activity in intrinsic cardiac neurons (Armour, 1999, Horackova et al., 1999, Thompson et al., 2000). Cardiac stimuli, transduced by ICG sensory neurons, could be transmitted to ICG local circuit or efferent neurons by synaptic glutamate release for modulation of each cardiac cycle (Armour, 1999, 2008). The majority of cholinergic postganglionic neurons project to the cardiac conducting system, but direct cholinergic innervation of the cardiac myocardium does occur from ICG postganglionic neurons, although the innervation of rat atrial myocardium is much greater than ventricular myocardium (Schafer et al., 1998). An autonomic efferent neuron projection from the ICG to the ventricles should not be ruled out from the retrogradely labeled neurons identified in the current study.
Both inotropic (iGluRs) and metabotropic glutamate (mGluRs) receptors in the rat intrinsic cardiac nervous system have been demonstrated (Gill et al., 1998, 1999). ICG neurons nerve terminals contain immunoreactivity for iGluRs including GluR 2/3, KA2, and NR1. Among mGluRs, mGluR5 is present predominantly in ventricles, whereas mGluR1A and mGluR2/3 are in the atrium (Gill et al., 1998, 1999). iGluRs may be used for modulating cardiac contractility and rhythmicity, whereas mGluRs may be used for long term cellular control by second messenger systems (Gill et al., 1998, 1999). Injection of glutamate into the ICG induces cardiac index changes including heart rate and ventricular chamber systolic pressure and these changes are preserved after acute and chronic decentralization (Huang et al., 1993a, Thompson et al., 2000). With the results from previous reports and the current study, we propose that glutamate functions as a neurotransmitter for some neurons of the intrinsic cardiac nervous system to contribute to regional cardiac control.
The intrinsic cardiac ganglia have an essential role in maintaining and modulating cardiac function. For example, in transplanted hearts, the ICG neurons have been shown to help in maintaining cardiac ionotropism and chronotropism (Murphy et al., 1994). In both acute and chronic decentralization studies, independent neuronal activities are observed consistently in ICG neurons and mechanical or chemical stimulation of the ventricle changes the ICG neuronal activities and causes changes in cardiac indices (King and Coakley, 1958, Pardini et al., 1987, Gagliardi et al., 1988, Armour and Hopkins, 1990, Ardell et al., 1991, Huang et al., 1993a, Huang et al., 1993b, Pauza et al., 1997, Thompson et al., 2000, Armour, 2004, 2008). These studies indicate an independent afferent input that consistently contributes to regional cardiac control. A working model for the intrinsic cardiac nervous system has been proposed with sensory, local circuit, and autonomic efferent neurons coordinating and cooperating in a neuronal hierarchy for cardiac modulation (Ardell et al., 1991, Armour, 1999, 2008). In this model, sensory afferent information from intrinsic sensory neurons would be able to modify cardiac rate and regional contractile force in a “beat to beat fashion” (Armour, 1999, Thompson et al., 2000, Armour, 2008). Our current report illustrates that a large population of ICG neurons are neurochemically glutamatergic in nature. These neurons express GLS, the synthetic enzyme for glutamate production, and VGLUT1 and/or VGLUT2, synaptic vesicle transporters for glutamate neurotransmission. These may be previously undescribed glutamatergic autonomic neurons, since many were cholinergic, expressing VAChT. A subpopulation of glutamatergic ICG neurons were identified by retrograde tracing from the ventricles and may fulfill a sensory role providing the ICG with venticular mechanical or environmental changes. Studies examining the expression of sensory transducer proteins in ICG neurons might address the sensory nature of some these glutamatergic ICG neurons that project to the ventricular wall. Furthermore, evaluation of glutamatergic ICG neurons may be important in understanding the ICG’s role in “beat to beat” modulation of cardiac function and its response to pathological conditions, such as hypertension and atrial arrhythmia.
Highlights.
Rat ICG neurons contain glutaminase, synthetic enzyme for glutamate, and VGLUT1&2, glutamate vesicular transporters.
Some atrial ICG neurons send axons to the ventricular wall and express glutaminase and VGLUT1&2.
Retrogradely labeled ICG neurons were larger than other glutamatergic ICG neurons.
Many glutamatergic ICG neurons also were cholinergic, expressing VAChT.
VGLUT1 and VGLUT2 co-localization occurred in ICG neurons, but with variation in their protein expression level.
Acknowledgments
This work was supported by National Institutes of Health grant NIH AR47410.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflicts of interest in this study.
References
- Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am J Physiol. 1991;260:H713–H721. doi: 10.1152/ajpheart.1991.260.3.H713. [DOI] [PubMed] [Google Scholar]
- Ardell JL, Randall WC, Cannon WJ, Schmacht DC, Tasdemiroglu E. Differential sympathetic regulation of automatic, conductile, and contractile tissue in dog heart. Am J Physiol. 1988;255:H1050–H1059. doi: 10.1152/ajpheart.1988.255.5.H1050. [DOI] [PubMed] [Google Scholar]
- Armour JA. Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res. 1999;41:41–54. doi: 10.1016/s0008-6363(98)00252-1. [DOI] [PubMed] [Google Scholar]
- Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol. 2004;287:R262–R271. doi: 10.1152/ajpregu.00183.2004. [DOI] [PubMed] [Google Scholar]
- Armour JA. Potential clinical relevance of the 'little brain' on the mammalian heart. Exp Physiol. 2008;93:165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- Armour JA, Collier K, Kember G, Ardell JL. Differential selectivity of cardiac neurons in separate intrathoracic autonomic ganglia. Am J Physiol. 1998;274:R939–R949. doi: 10.1152/ajpregu.1998.274.4.R939. [DOI] [PubMed] [Google Scholar]
- Armour JA, Hopkins DA. Activity of canine in situ left atrial ganglion neurons. Am J Physiol. 1990;259:H1207–H1215. doi: 10.1152/ajpheart.1990.259.4.H1207. [DOI] [PubMed] [Google Scholar]
- Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Aschoff A, Hollander H. Fluorescent compounds as retrograde tracers compared with horseradish peroxidase (HRP). I. A parametric study in the central visual system of the albino rat. J Neurosci Methods. 1982;6:179–197. doi: 10.1016/0165-0270(82)90083-8. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195:183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- Bolt BR. Ph.D. Dissertation. Stillwater, OK, USA: Oklahoma State University; 2016. The role of aspartate aminotransferase during inflammation-induced peripheral nociception. [Google Scholar]
- Brumovsky P, Watanabe M, Hokfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147:469–490. doi: 10.1016/j.neuroscience.2007.02.068. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Robinson DR, La JH, Seroogy KB, Lundgren KH, Albers KM, Kiyatkin ME, Seal RP, Edwards RH, Watanabe M, Hokfelt T, Gebhart GF. Expression of vesicular glutamate transporters type 1 and 2 in sensory and autonomic neurons innervating the mouse colorectum. J Comp Neurol. 2011;519:3346–3366. doi: 10.1002/cne.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Powley TL. Nucleus ambiguus projections to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol. 2000;424:588–606. [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, Doyle FJ., 3rd Projections of the dorsal motor nucleus of the vagus to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol. 1999;410:320–341. [PubMed] [Google Scholar]
- Corbett EK, Sinfield JK, McWilliam PN, Deuchars J, Batten TF. Differential expression of vesicular glutamate transporters by vagal afferent terminals in rat nucleus of the solitary tract: projections from the heart preferentially express vesicular glutamate transporter 1. Neuroscience. 2005;135:133–145. doi: 10.1016/j.neuroscience.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Crosby HA, Ihnat M, Spencer D, Miller KE. Expression of Glutaminase and Vesicular Glutamate Transporter Type 2 Immunoreactivity in Rat Sacral Dorsal Root Ganglia Following a Surgical Tail Incision. Pharm Pharmacol Int J. 2015;2(3):00023. doi: 10.15406/ppij.2015.02.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Klemm MF, Steele PA. Different types of ganglion cell in the cardiac plexus of guinea-pigs. J Physiol. 1995;486(Pt 2):453–471. doi: 10.1113/jphysiol.1995.sp020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Zaleska MM, Nelson D, Nissim I, Yudkoff M. Neuronal glutamine utilization: glutamine/glutamate homeostasis in synaptosomes. J Neurochem. 1990;54:2057–2069. doi: 10.1111/j.1471-4159.1990.tb04911.x. [DOI] [PubMed] [Google Scholar]
- Ewald P, Neuhuber WL, Raab M. Vesicular glutamate transporter 1 immunoreactivity in extrinsic and intrinsic innervation of the rat esophagus. Histochem Cell Biol. 2006;125:377–395. doi: 10.1007/s00418-005-0083-z. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gagliardi M, Randall WC, Bieger D, Wurster RD, Hopkins DA, Armour JA. Activity of in vivo canine cardiac plexus neurons. Am J Physiol. 1988;255:H789–H800. doi: 10.1152/ajpheart.1988.255.4.H789. [DOI] [PubMed] [Google Scholar]
- Gatti PJ, Johnson TA, Phan P, Jordan IK, 3rd, Coleman W, Massari VJ. The physiological and anatomical demonstration of functionally selective parasympathetic ganglia located in discrete fat pads on the feline myocardium. J Auton Nerv Syst. 1995;51:255–259. doi: 10.1016/0165-1838(94)00139-b. [DOI] [PubMed] [Google Scholar]
- Gill SS, Pulido OM, Mueller RW, McGuire PF. Molecular and immunochemical characterization of the ionotropic glutamate receptors in the rat heart. Brain Res Bull. 1998;46:429–434. doi: 10.1016/s0361-9230(98)00012-4. [DOI] [PubMed] [Google Scholar]
- Gill SS, Pulido OM, Mueller RW, McGuire PF. Immunochemical localization of the metabotropic glutamate receptors in the rat heart. Brain Res Bull. 1999;48:143–146. doi: 10.1016/s0361-9230(98)00154-3. [DOI] [PubMed] [Google Scholar]
- Herzog E, Landry M, Buhler E, Bouali-Benazzouz R, Legay C, Henderson CE, Nagy F, Dreyfus P, Giros B, El Mestikawy S. Expression of vesicular glutamate transporters, VGLUT1 and VGLUT2, in cholinergic spinal motoneurons. Eur J Neurosci. 2004;20:1752–1760. doi: 10.1111/j.1460-9568.2004.03628.x. [DOI] [PubMed] [Google Scholar]
- Hochman S, Schmidt BJ. Whole cell recordings of lumbar motoneurons during locomotor-like activity in the in vitro neonatal rat spinal cord. J Neurophysiol. 1998;79:743–752. doi: 10.1152/jn.1998.79.2.743. [DOI] [PubMed] [Google Scholar]
- Hoffman EM, Schechter R, Miller KE. Fixative composition alters distributions of immunoreactivity for glutaminase and two markers of nociceptive neurons, Nav1.8 and TRPV1, in the rat dorsal root ganglion. J Histochem Cytochem. 2010;58:329–344. doi: 10.1369/jhc.2009.954008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EM, Zhang Z, Anderson MB, Schechter R, Miller KE. Potential mechanisms for hypoalgesia induced by anti-nerve growth factor immunoglobulin are identified using autoimmune nerve growth factor deprivation. Neuroscience. 2011;193:452–465. doi: 10.1016/j.neuroscience.2011.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EM, Zhang Z, Schechter R, Miller KE. Glutaminase Increases in Rat Dorsal Root Ganglion Neurons after Unilateral Adjuvant-Induced Hind Paw Inflammation. Biomolecules. 2016;6(1):10. doi: 10.3390/biom6010010. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DB, Shepherd AV, Southerland EM, Armour JA, Ardell JL. Neurochemical diversity of afferent neurons that transduce sensory signals from dog ventricular myocardium. Auton Neurosci. 2008;141:38–45. doi: 10.1016/j.autneu.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horackova M, Armour JA, Byczko Z. Distribution of intrinsic cardiac neurons in whole-mount guinea pig atria identified by multiple neurochemical coding. A confocal microscope study. Cell Tissue Res. 1999;297:409–421. doi: 10.1007/s004410051368. [DOI] [PubMed] [Google Scholar]
- Horackova M, Slavikova J, Byczko Z. Postnatal development of the rat intrinsic cardiac nervous system: a confocal laser scanning microscopy study in whole-mount atria. Tissue Cell. 2000;32:377–388. doi: 10.1054/tice.2000.0126. [DOI] [PubMed] [Google Scholar]
- Huang MH, Smith FM, Armour JA. Amino acids modify activity of canine intrinsic cardiac neurons involved in cardiac regulation. Am J Physiol. 1993a;264:H1275–H1282. doi: 10.1152/ajpheart.1993.264.4.H1275. [DOI] [PubMed] [Google Scholar]
- Huang MH, Sylven C, Pelleg A, Smith FM, Armour JA. Modulation of in situ canine intrinsic cardiac neuronal activity by locally applied adenosine, ATP, or analogues. Am J Physiol. 1993b;265:R914–R922. doi: 10.1152/ajpregu.1993.265.4.R914. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Burette A, Rustioni A, Valtschanoff JG. Vanilloid receptor VR1-positive primary afferents are glutamatergic and contact spinal neurons that co-express neurokinin receptor NK1 and glutamate receptors. J Neurocytol. 2004;33:321–329. doi: 10.1023/B:NEUR.0000044193.31523.a1. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O, Tresch MC, Harris-Warrick RM. Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord. Brain Res Bull. 2000;53:649–659. doi: 10.1016/s0361-9230(00)00398-1. [DOI] [PubMed] [Google Scholar]
- King TS, Coakley JB. The intrinsic nerve cells of the cardiac atria of mammals and man. J Anat. 1958;92:353–376. [PMC free article] [PubMed] [Google Scholar]
- Kobbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Kukanova B, Mravec B. Complex intracardiac nervous system. Bratisl Lek Listy. 2006;107:45–51. [PubMed] [Google Scholar]
- Lagerstrom MC, Rogoz K, Abrahamsen B, Lind AL, Olund C, Smith C, Mendez JA, Wallen-Mackenzie A, Wood JN, Kullander K. A sensory subpopulation depends on vesicular glutamate transporter 2 for mechanical pain, and together with substance P, inflammatory pain. Proc Natl Acad Sci U S A. 2011;108:5789–5794. doi: 10.1073/pnas.1013602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M, Bouali-Benazzouz R, El Mestikawy S, Ravassard P, Nagy F. Expression of vesicular glutamate transporters in rat lumbar spinal cord, with a note on dorsal root ganglia. J Comp Neurol. 2004;468:380–394. doi: 10.1002/cne.10988. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ. Neurotransmitter mechanisms of rat vagal afferent neurons. Clin Exp Pharmacol Physiol. 1995;22:869–873. doi: 10.1111/j.1440-1681.1995.tb01953.x. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. L-glutamate as a neurotransmitter at baroreceptor afferents: evidence from in vivo microdialysis. Neuroscience. 1994;58:585–591. doi: 10.1016/0306-4522(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Li JL, Xiong KH, Dong YL, Fujiyama F, Kaneko T, Mizuno N. Vesicular glutamate transporters, VGluT1 and VGluT2, in the trigeminal ganglion neurons of the rat, with special reference to coexpression. J Comp Neurol. 2003;463:212–220. doi: 10.1002/cne.10755. [DOI] [PubMed] [Google Scholar]
- Liu MT, Rothstein JD, Gershon MD, Kirchgessner AL. Glutamatergic enteric neurons. J Neurosci. 1997;17:4764–4784. doi: 10.1523/JNEUROSCI.17-12-04764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malet M, Brumovsky PR. VGLUTs and Glutamate Synthesis-Focus on DRG Neurons and Pain. Biomolecules. 2015;5:3416–3437. doi: 10.3390/biom5043416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malet M, Vieytes CA, Lundgren KH, Seal RP, Tomasella E, Seroogy KB, Hokfelt T, Gebhart GF, Brumovsky PR. Transcript expression of vesicular glutamate transporters in lumbar dorsal root ganglia and the spinal cord of mice - effects of peripheral axotomy or hindpaw inflammation. Neuroscience. 2013;248:95–111. doi: 10.1016/j.neuroscience.2013.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B, Arvidsson U, Zhang X, Jacobsson G, Villar MJ, Hokfelt T. Glutamate transporter mRNA and glutamate-like immunoreactivity in spinal motoneurones. Neuroreport. 1993;5:337–340. doi: 10.1097/00001756-199312000-00040. [DOI] [PubMed] [Google Scholar]
- Miller KE, Balbas JC, Benton RL, Lam TS, Edwards KM, Kriebel RM, Schechter R. Glutaminase immunoreactivity and enzyme activity is increased in the rat dorsal root ganglion following peripheral inflammation. Pain Res Treat. 2012;2012:414697. doi: 10.1155/2012/414697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Douglas VD, Kaneko T. Glutaminase immunoreactive neurons in the rat dorsal root ganglion contain calcitonin gene-related peptide (CGRP) Neurosci Lett. 1993;160:113–116. doi: 10.1016/0304-3940(93)90926-c. [DOI] [PubMed] [Google Scholar]
- Miller KE, Hoffman EM, Sutharshan M, Schechter R. Glutamate pharmacology and metabolism in peripheral primary afferents: physiological and pathophysiological mechanisms. Pharmacol Ther. 2011;130:283–309. doi: 10.1016/j.pharmthera.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Richards BA, Kriebel RM. Glutamine-, glutamine synthetase-, glutamate dehydrogenase- and pyruvate carboxylase-immunoreactivities in the rat dorsal root ganglion and peripheral nerve. Brain Res. 2002;945:202–211. doi: 10.1016/s0006-8993(02)02802-0. [DOI] [PubMed] [Google Scholar]
- Moravec M, Moravec J. 3-D characterization of ganglion cells of the terminal nerve plexus of rat atrioventricular junction. J Auton Nerv Syst. 1998;74:1–12. doi: 10.1016/s0165-1838(98)00118-0. [DOI] [PubMed] [Google Scholar]
- Murphy DA, O'Blenes S, Hanna BD, Armour JA. Capacity of intrinsic cardiac neurons to modify the acutely autotransplanted mammalian heart. J Heart Lung Transplant. 1994;13:847–856. [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Pardini BJ, Patel KP, Schmid PG, Lund DD. Location, distribution and projections of intracardiac ganglion cells in the rat. J Auton Nerv Syst. 1987;20:91–101. doi: 10.1016/0165-1838(87)90106-8. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Pauziene N, Pakeltyte G, Stropus R. Comparative quantitative study of the intrinsic cardiac ganglia and neurons in the rat, guinea pig, dog and human as revealed by histochemical staining for acetylcholinesterase. Ann Anat. 2002;184:125–136. doi: 10.1016/S0940-9602(02)80005-X. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Skripkiene G, Skripka V, Pauziene N, Stropus R. Morphological study of neurons in the nerve plexus on heart base of rats and guinea pigs. J Auton Nerv Syst. 1997;62:1–12. doi: 10.1016/s0165-1838(96)00102-6. [DOI] [PubMed] [Google Scholar]
- Raab M, Neuhuber WL. Vesicular glutamate transporter 2 immunoreactivity in putative vagal mechanosensor terminals of mouse and rat esophagus: indication of a local effector function? Cell Tissue Res. 2003;312:141–148. doi: 10.1007/s00441-003-0721-5. [DOI] [PubMed] [Google Scholar]
- Raab M, Neuhuber WL. Glutamatergic functions of primary afferent neurons with special emphasis on vagal afferents. Int Rev Cytol. 2007;256:223–275. doi: 10.1016/S0074-7696(07)56007-9. [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Grkovic I, Anderson CR. Immunohistochemical analysis of intracardiac ganglia of the rat heart. Cell Tissue Res. 2003;314:337–350. doi: 10.1007/s00441-003-0805-2. [DOI] [PubMed] [Google Scholar]
- Rysevaite K, Saburkina I, Pauziene N, Vaitkevicius R, Noujaim SF, Jalife J, Pauza DH. Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations. Heart Rhythm. 2011;8:731–738. doi: 10.1016/j.hrthm.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience. 1998;84:361–376. doi: 10.1016/s0306-4522(97)80196-0. [DOI] [PubMed] [Google Scholar]
- Selyanko AA. Membrane properties and firing characteristics of rat cardiac neurones in vitro. J Auton Nerv Syst. 1992;39:181–189. doi: 10.1016/0165-1838(92)90011-5. [DOI] [PubMed] [Google Scholar]
- Senba E, Kaneko T, Mizuno N, Tohyama M. Somato-, branchio- and viscero-motor neurons contain glutaminase-like immunoreactivity. Brain Res Bull. 1991;26:85–97. doi: 10.1016/0361-9230(91)90193-n. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2) J Neurosci. 2001;21:RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GW, Collier K, Ardell JL, Kember G, Armour JA. Functional interdependence of neurons in a single canine intrinsic cardiac ganglionated plexus. J Physiol. 2000;528:561–571. doi: 10.1111/j.1469-7793.2000.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ma J, Kirchgessner AL. Vesicular glutamate transporter 2 in the brain-gut axis. Neuroreport. 2001;12:3929–3934. doi: 10.1097/00001756-200112210-00015. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ES, Jin MU, Hong JH, Kim YS, Choi SY, Kim TH, Cho YS, Bae YC. Expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in the rat dental pulp and trigeminal ganglion following inflammation. PLoS One. 2014;9:e109723. doi: 10.1371/journal.pone.0109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara O, Matsuo A, Bellier JP, Aimi Y. Demonstration of choline acetyltransferase of a peripheral type in the rat heart. J Histochem Cytochem. 2007;55:287–299. doi: 10.1369/jhc.6A7092.2006. [DOI] [PubMed] [Google Scholar]