Abstract
Extremely interesting for aging research are those individuals able to reach older ages still with functions similar to those of younger counterparts. We examined liver samples from ad libitum-fed old (28-month-old, AL-28) and ad libitum-fed very old (32-month-old, AL-32) rats for a number of markers, relevant for mitochondrial functionality and mitochondrial DNA (mtDNA) content. As for the mtDNA content and the protein amounts of the citrate synthase and the antioxidant peroxiredoxin III there were no significant changes in the AL-32 animals. No significant longevity-related change was found for TFAM amount, but a 50% reduction in the amount of the Lon protease, responsible for turnover of TFAM inside mitochondria, characterized the AL-32 rats. No longevity-related change was observed also for the amounts of the mtDNA repair enzymes OGG1 and APE1, whereas the intra-mitochondrial amount of the cytochrome c protein showed a 50% increase in the AL-32 rats, indicating a likely reduced initiation of the intrinsic apoptotic pathway. Totally unexpected was the doubling of two proteins, very relevant for mitochondrial dynamics, namely MFN2 and DRP1, in the AL-32 rats. This prompted us to the calculation of all individual fusion indexes that grouped together in the AL-32 rats, while in the AL-28 animals were very different. We found a strong positive correlation between the fusion indexes and the respective mtDNA contents in two AL-28 and four AL-32 rats. This supports the idea that the limited prevalence of fusion above a still active fission should have ensured a functional mitochondrial network and should have led to a quite narrow range of high mtDNA contents, likely the best-suitable for extended longevity. Our findings strongly suggest that, among the multiple causes leading to the longevity of the AL-32 rats, the maintenance of an adult-like balance of mitochondrial dynamics seems to be very relevant for the regulation of mtDNA content and functionality.
Keywords: Long-living rats, Aged liver mitochondria, mtDNA and related proteins, Mitochondrial dynamics, Fusion index correlation with mtDNA content
1. Introduction
Rodents represent an important model in aging studies due to the quite short average lifespan of animals, which makes experimental analysis feasible in acceptable times of housing. Also, a huge amount of data about various aspects of the rodents aging process is already available. The long-living Fischer 344 × Brown Norway strain is very often chosen for such studies also because of its capability to reach 28 months of age and extend, although with a fairly high incidence of mortality, to the very considerable age of 32 months or more. The natural occurrence of a very long-living population makes this strain particularly suitable for the identification of molecular features possibly relevant in such extended longevity. The mitochondrial involvement in aging is a well-established point (Held and Houtkooper 2015; López-Lluch et al. 2015) as is its tissue-specificity (Hu and Liu 2014; Picca et al. 2014). Liver is metabolically very active and presents a marked age-related decrease in mitochondrial functionality and biogenesis that is recently undergoing a thorough molecular analysis (Mach et al. 2015; Picca et al. 2013a). To dissect some of the age-related mitochondrial effects we compared a number of markers, relevant for mitochondrial functionality and for mtDNA content, between a group of ad libitumfed old (28-month-old, AL-28) rats and a counterpart of ad libitumfed very old (32-month-old, AL-32) animals. Mitochondrial functionality was assayed through the determination of the amounts of citrate synthase and peroxiredoxin III (Prx III) proteins. MtDNA content was measured and, due to its substantial relationship with the amount of the histone-like protein mitochondrial transcription factor A (TFAM), we also compared the amounts of TFAM and of the Lon protease, involved in TFAM turnover, between the same groups of rats. To deepen the analysis at mtDNA level we determined the protein amounts of two DNA repair enzymes, namely APE1 and OGG1, and of two proteins active in mitochondrial dynamics, that are MFN2 and DRP1, in AL-28 and AL-32 rats. We also measured the amount of intra-mitochondrial cytochrome c in both groups of animals. Finally, some very intriguing conclusions highlighting the relevance of an “adult-like” balance of mitochondrial dynamics and of mtDNA content for the extended longevity of the assayed rats could be inferred. In fact, present findings suggest that the age-related prevalence of fusion above fission might have been slowed in the AL-32 rats leading to an improved maintenance of mtDNA and contributing to their longevity.
2. Materials and methods
2.1. Samples
The study was approved by the Institutional Animal Care and Use Committee at the University of Florida. All procedures were performed in accordance with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals. Liver samples used in this study were from Fischer 344 × Brown Norway (F344BNF1) male rats obtained from the National Institute of Aging colony (Indianapolis, IN) and housed at the Department of Aging and Geriatric Research, Division of Biology of Aging, College of Medicine, University of Florida, Gainesville, FL (USA). The animals consisted of the following groups: 28-month-old ad libitum-fed (AL-28, n = 6) and 32-month-old ad libitum-fed (AL-32, n = 6) rats. The initial size of the age-groups was larger than the final one to compensate for intervening natural mortality. However, the number of six animals/group appeared sufficient for the study in consideration of the accurate evaluations described in (Hagen et al. 2004), based on the survival curves of specific rat strains including the F344BNF1(Turturro et al. 1999). Animals were anesthetized before being sacrificed and samples from the liver were immediately removed, snap-frozen in isopentane cooled by liquid nitrogen, and stored in liquid nitrogen until further analysis.
2.2. Determination of mtDNA content
MtDNA content was measured using quantitative real time polymerase chain reaction (qRT-PCR). RT-PCR reactions were performed via SYBR Green chemistry in Fast mode on a QuantStudio™ 7 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The primers were specific, respectively, for the rat mitochondrial D-loop region (numbering is according to GenBank™ accession number AY172581) and for the rat nuclear β-actin gene (numbering is according to GenBank™ accession number VO1217.1) and are listed in Table 1. The method has been validated by primer-limiting experiments and by evaluating the equal reaction efficiency of the two amplicons. Amplification specificity was controlled by melting curve analysis and gel electrophoresis. Each sample was analyzed in triplicate in 20 μl of final volume containing: iTaq SYBR Green Supermix PCR 1X Master Mix (Bio-Rad Laboratories Inc., Hercules, CA, USA), 0.2 μM forward and reverse primers, and DNA template (25 ng). After 5 min of denaturation at 95 °C, amplification proceeded for 40 cycles, each consisting of denaturation at 95 °C for 3 s, annealing, and extension at 60 °C for 30 s. The quantification of the mtDNA content in all rats, normalized to the respective β-actin, was performed according to the Pfaffl mathematical model (Pfaffl 2001).
Table 1.
Oligonucleotide Primers sequences.
| Primers for RT-PCR | ||||
|---|---|---|---|---|
|
| ||||
| Primer set | Forward primer | Reverse primer | (nps) | (nps) |
| mtDNA For/mtDNA Rev | 5′-GGTTCTTACTTCAGGGCCATCA-3′ | 5′–TGATTAGACCCGTTACCATCGA-3′ | (15,785–15,806) | (15,868–15,847) |
| Beta-actin For/Beta-actin Rev | 5′–CCCAGCCATGTACGTAGCCA–3′ | 5′–CGTCTCCGGAGTCCATCAC–3′ | (2181–2200) | (2266–2248) |
2.3. Western blotting
Mitochondrial isolation was performed as follows. Approximately half of the liver from each of the AL-28 and AL-32 animals was homogenized with a Potter–Elvehjem homogenizer (four strokes in 1 min at 500 rpm) in an isolation medium consisting of 220 mM mannitol, 70 mM sucrose, 20 mM Tris–HCl, 1 mM EDTA, and 5 mM EGTA, pH 7.4 at 4 °C. The homogenate was centrifuged at 500 ×g for 10 min at 4 °C and the pellet consisting of nuclei and unbroken cells was discarded; the resulting supernatant was centrifuged at 8500 ×g for 10 min at 4 °C and the related pellet was washed twice before being resuspended in isolation medium with the addition of a cocktail of protease inhibitors (cOmplete™ ULTRA Tablets, Mini, EDTA-free, Roche Diagnostics, Mannheim, Germany). Proteins were quantified with the Bradford method (Bio-Rad Laboratories Inc., Hercules, CA, USA) according to the supplier’s instructions. 10 μg of mitochondrial proteins were used for western blot analysis as described in (Picca et al. 2014) with the following primary antibodies dilutions used: TFAM (1:50,000), VDAC (1:50,000, Abcam), OGG1 (1:2500, Abcam), APE1 (1:5000, Abcam), MFN2 (1:5000, Abnova), DRP1 (1:2500, Abnova), Cyt c (1:500, Pharmingen), Citrate Synthase (1:100,000, Santa Cruz), Lon Protease (1:10,000) and Prx III (1:50,000, AbFrontier). The antibodies against TFAM and Lon were custom-made and kindly donated, respectively, by Dr. H. Hinagaki (Department of Chemistry, National Industrial Research Institute of Nagoya, Japan) and Dr. C. Suzuki (Department of Biochemistry and Molecular Biology, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Newark, New Jersey, USA).
2.4. Statistical analysis
The statistical significance of differences between the two groups of animals was assessed by t-test analysis with the SPSS Base 11.5 software (SPSS Inc., Chicago, IL, USA) assuming the samples as independent. Statistical significance for tests was set at p < 0.05 or p < 0.01. Correlation between variables was analyzed using Pearson’s test. Statistical significance for the test was set at p < 0.05.
3. Results
3.1. Longevity-related effects on mtDNA content, citrate synthase and peroxiredoxin III amounts
In all tested rat tissues aging implies a statistically significant loss of mtDNA, although the entity of such loss is tissue-specific (Kang et al. 2013; Pesce et al. 2005; Picca et al. 2014). The availability of liver samples from very long-lasting rats (AL-32), allowed us to measure their relative mtDNA content as well as to assay their mitochondrial functionality through determination of the amounts of two proteins crucial for the organelle metabolism namely the citrate synthase (Wredenberg et al. 2002) and the antioxidant Prx III (Chang et al. 2004; Lee et al. 2014). The counterpart in the comparison included samples from less old animals (AL-28), clearly affected by the aging process as demonstrated in a previous study by our group (Picca et al. 2013a). The relative mtDNA content was measured by quantitative Real Time-PCR and the results for both age groups are reported in Fig. 1 that shows the absence of any statistically significant change with increasing age. Then, we turned to determine, by western blots experiments, the protein amount of the first fundamental enzyme of the tricarboxylic acids cycle that is citrate synthase, whose activity is so relevant for mitochondrial metabolism and survival to be used as a marker of mitochondrial mass (Betik et al. 2009). In the same experiments the protein from the outer mitochondrial membrane VDAC was used as internal standard for loading equal amounts from all tested samples. The results of the comparison between the two groups of rats are presented in Fig. 2 showing no significant changes in the AL-32 animals with respect to the less aged counterparts. Analyzing, also by western blots experiments, the amount of the scavenging mitochondrial protein Prx III, we found, as reported in Fig. 2, no significant changes in the older rats (AL-32) that paralleled the citrate synthase trend.
Fig. 1.
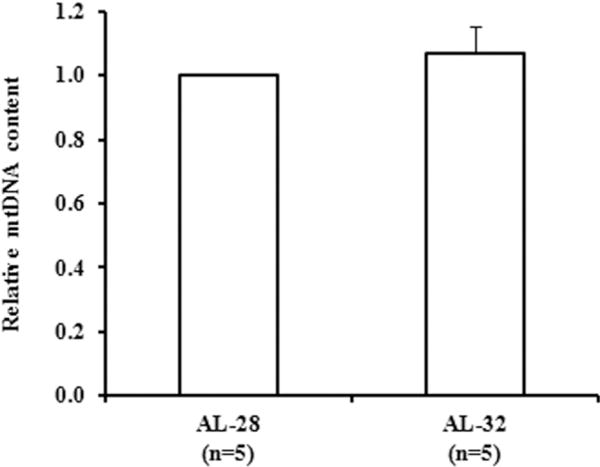
Effect of extended aging on mtDNA content in rat liver. Extended aging did not induce any change in the mtDNA content. The histogram shows the mean value of the ratio mtDNA/nuclear DNA, determined by qRT-PCR, in AL-32 rats compared to AL-28 rats. Bars represent the mean (±SE) obtained, respectively, from analysis in triplicate of total nucleic acids from each AL-28 and AL-32 rat. The comparison was made with respects to the value of the AL-28 rats fixed as 1. n = number of analyzed animals.
Fig. 2.
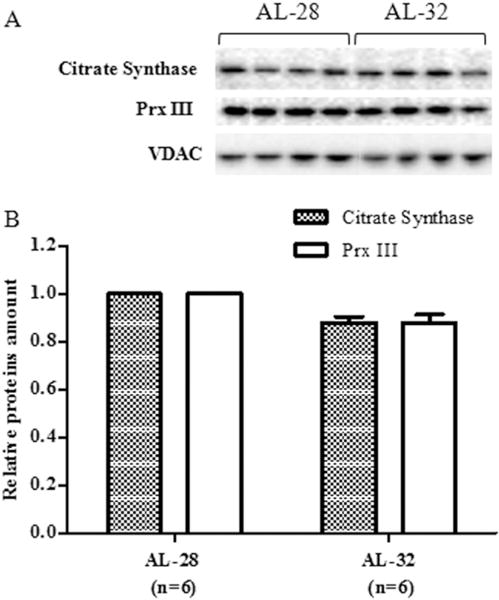
Effect of extended aging on citrate synthase and peroxiredoxin III (Prx III) amounts in rat liver. Extended aging did not induce any significant change in citrate synthase and Prx III amounts. A. Representative western blot carried out in four rats from each assayed group. The bands from top to bottom show, respectively, the signals from citrate synthase, Prx III and VDAC. B. The histogram shows the relative amounts of citrate synthase and Prx III in AL-32 rats, determined by densitometry analysis of the results from triplicated western blots experiments, compared to AL-28 rats. The densitometric value of OD units of every citrate synthase and Prx III band was related to the number of OD units of the respective band of VDAC for each analyzed sample. Bars represent the mean ((±SE) of the relative (citrate synthase/VDAC, Prx III/VDAC) values obtained from each AL-28 and AL-32 rat. Comparisons were made with respects to the value of the AL-28 rats, fixed as 1. n = number of analyzed animals.
3.2. Longevity-related effects on TFAM and Lon protease
Having verified the absence of effects of extended aging on mtDNA content and metabolically relevant proteins, we analyzed the amounts of the histone-like protein of mtDNA that is TFAM (Picca and Lezza 2015) and of the intra-mitochondrial protease responsible for its turn-over namely Lon (Matsushima et al. 2010). TFAM is involved in multiple functions that are performed in close connection with mtDNA molecules. We determined, by western blot experiments, the amount of TFAM in both groups of rats and in the same experiments the protein from the outer mitochondrial membrane VDAC was used as internal standard for loading equal amounts from all tested samples. The results are presented in Fig. 3 and no statistically significant longevity-related change was found for TFAM. On the other hand, the amount of the protease Lon (Fig. 3) showed a 50% statistically significant decrease in the AL-32 rats with respects to the less aged counterparts.
Fig. 3.
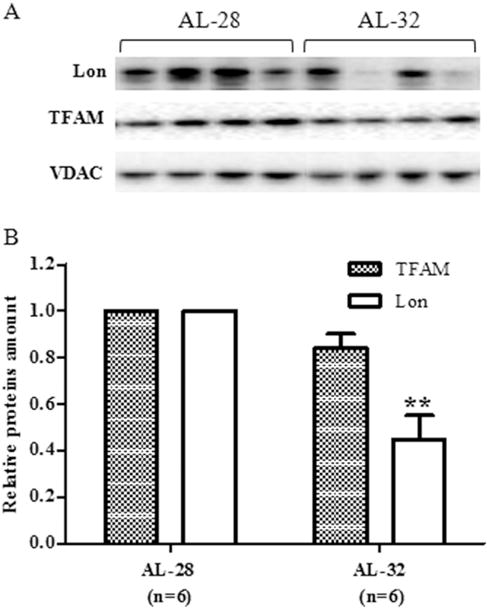
Effect of extended aging on TFAM and Lon amounts in rat liver. Extended aging did not induce any significant change in TFAM amount and a 50% decrease in Lon amount. A. Representative western blot carried out in four rats from each assayed group. The bands from top to bottom show, respectively, the signals from Lon, TFAM and VDAC. B. The histogram shows the relative amounts of TFAM and Lon in AL-32 rats, determined by densitometry analysis of the results from triplicated western blots experiments, compared to AL-28 rats. The densitometric value of OD units of every TFAM and Lon band was related to the number of OD units of the respective band of VDAC for each analyzed sample. Bars represent the mean ((±SE) of the relative (TFAM/VDAC, Lon/VDAC) values obtained from each AL-28 and AL-32 rat. Comparisons were made with respects to the value of the AL-28 rats, fixed as 1. **p < 0.01 versus the value of the AL-28 rats; n = number of analyzed animals.
3.3. Longevity-related effects on enzymes involved in mtDNA repair and cytochrome c
Considering the maintenance, in the AL-32 rats, of mtDNA content as well as of TFAM, citrate synthase and Prx III amounts, we decided to evaluate the protein amount of 8-Oxoguanine glycosylase (OGG1) and of apurinic/apyrimidinic endonuclease 1 (APE1), two enzymes involved in the base excision repair (BER) that is the prevalent DNA repair mechanism inside mitochondria. In such multi-steps pathway a modified base as 8-oxo-7,8-dihydroguanine(8-oxo-G) is identified by the specific OGG1 and removed, leaving an abasic site that is recognized by APE1, which removes the abasic ribose and permits the complete repair of DNA by DNA polymerase γ (POLG) and ligase III (Scheibye-Knudsen et al. 2015). The amounts of OGG1 and APE1 proteins were determined, by western blot experiments, in both groups of rats using the amount of the respective VDAC protein as the internal loading standard for each sample. The results, reported in Fig. 4, demonstrated the absence of any statistically significant longevity-related change for both enzymes, although there was a not significant 10% increase in the AL-32 amount of APE1. The absence of a longevity-related decrease in both mitochondrial repair enzymes suggested that, probably, the whole process of mtDNA repair in the AL-32 rats was not reduced with respects to the less aged counterparts. Therefore, it was very likely that the overall mtDNA damage, along with other well-known markers of the age-related mitochondrial oxidative stress, was not increased in the older animals, thus contributing to their extended longevity. This drove us to examine one of the major triggers of the mitochondrial apoptotic pathway that is the release of cytochrome c from mitochondria (Scorrano 2009). As shown in Fig. 4, the intra-mitochondrial amount of the cytochrome c protein showed a statistically significant longevity-related 50% increase in the AL-32 rats with respects to the AL-28 animals.
Fig. 4.
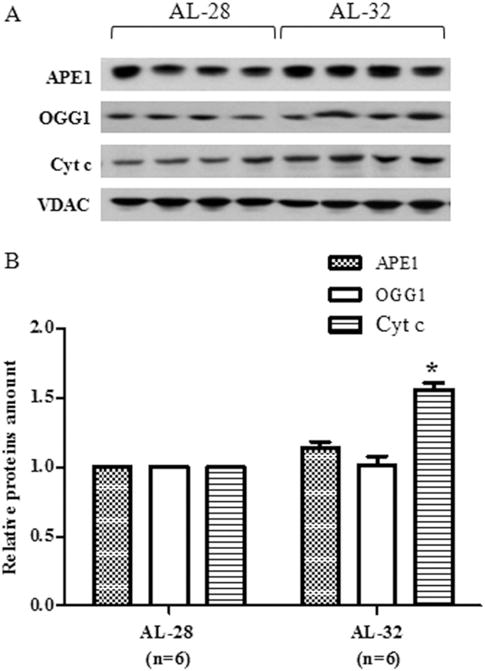
Effect of extended aging on OGG1, APE1 and cytochrome c (Cyt c) amounts in rat liver. Extended aging did not induce any change in OGG1 and APE1 amounts and induced a 50% increase in Cyt c amount. A. Representative western blot carried out in four rats from each assayed group. The bands from top to bottom show, respectively, the signals from APE1, OGG1, Cyt c and VDAC. B. The histogram shows the relative amounts of APE1, OGG1 and Cyt c in AL-32 rats, determined by densitometry analysis of the results from triplicated western blots experiments, compared to AL-28 rats. The densitometric value of OD units of every APE1, OGG1 and Cyt c band was related to the number of OD units of the respective band of VDAC for each analyzed sample. Bars represent the mean ((±SE) of the relative (APE1/VDAC, OGG1/VDAC, Cyt c/VDAC) values obtained from each AL-28 and AL-32 rat. Comparisons were made with respects to the value of the AL-28 rats, fixed as 1. *p < 0.05 versus the value of the AL-28 rats; n = number of analyzed animals.
3.4. Longevity-related effects on proteins involved in mitochondrial dynamics
To widen our survey of mitochondrial markers that might be linked to rat longevity, we examined two proteins relevant in the balance of mitochondrial dynamics namely the membrane-bound GTPase Mitofusin 2, (MFN2), required by fusion, and the cytosolic GTPase Dynamin-related protein 1 (DRP1), necessary for mitochondrial fission (Kasahara and Scorrano 2014). The amounts of the two proteins were determined, by western blot experiments, in the mitochondrial extracts of both groups using the amount of the respective VDAC protein as the internal loading standard for each sample. The results, presented in Fig. 5, demonstrated an unexpected, statistically significant doubling of both proteins in the AL-32 rats with respects to the AL-28 animals. This led to the calculation of the individual fusion indexes, by dividing each individual MFN2 amount for its respective DRP1 counterpart (Leduc-Gaudet et al. 2015), that resulted much closer to each other in the older rats group than those of the AL-28 animals (Table 2). Being the increase in both MFN2 and DRP1 amounts the most relevant change, as for entity size, and likely affecting the overall functionality of the crucial mitochondrial dynamics, it prompted us to verify the eventual relationship of the fusion index with the mtDNA content in all individual assayed animals. The results are presented in Fig. 6 where the individual fusion indexes were plotted in function of the respective mtDNA contents. It is noteworthy that all fusion indexes from the AL-32 rats were included between 0.88 and 1.92, suggesting that such a range might be well compatible with longevity. It was absolutely novel that two AL-28 rats and four out of the five AL-32 rats presented a statistically very significant, positive correlation (p = 0.0045, correlation coefficient 0.8931) between the assayed values. The outlier (0.88; 1649.09) from the AL-32 group was characterized by the lowest fusion index and by the highest value of mtDNA content, suggesting that it might represent a sort of best suitable candidate for longevity extending further than 32 months. This might be true also for the AL-28 rat featuring the highest mtDNA content (1593.18) in its group and presenting, similarly, the lowest fusion index (0.63). The points from the two remaining AL-28 animals were scattered representing rats whose fusion indexes were the highest values (3.26 and 2.44) and corresponded to mtDNA contents on average smaller than those of the other age-matched counterparts.
Fig. 5.
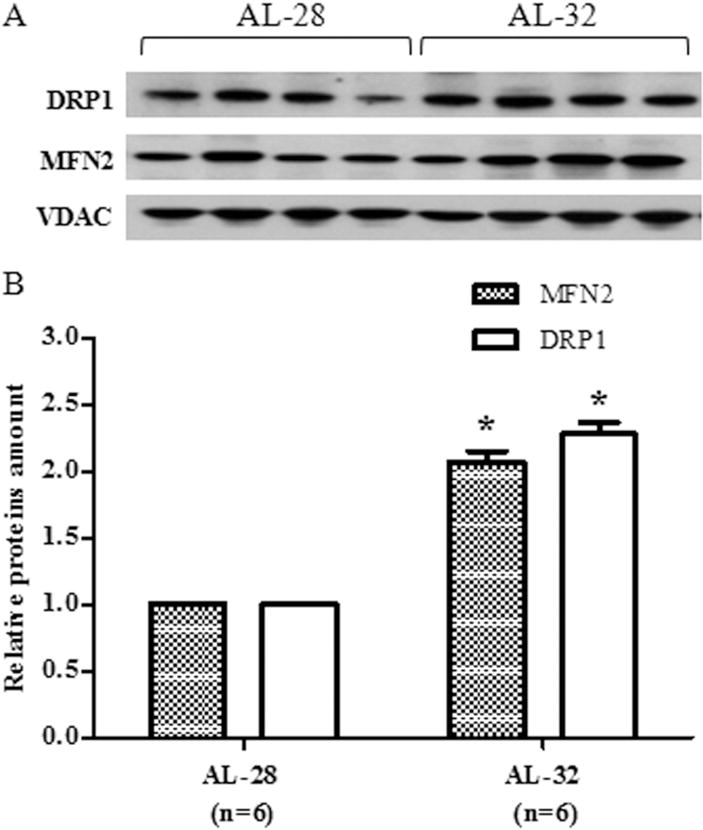
Effect of extended aging on MFN2 and DRP1 amounts in rat liver. Extended aging induced a doubling in MFN2 and DRP1 amounts. A. Representative western blot carried out in four rats from each assayed group. The bands from top to bottom show, respectively, the signals from DRP1, MFN2 and VDAC. B. The histogram shows the relative amounts of DRP1 and MFN2 in AL-32 rats, determined by densitometry analysis of the results from triplicated western blots experiments, compared to AL-28 rats. The densitometric value of OD units of every DRP1 and MFN2 band was related to the number of OD units of the respective band of VDAC for each analyzed sample. Bars represent the mean ((±SE) of the relative (DRP1/VDAC, MFN2/VDAC) values obtained from each AL-28 and AL-32 rat. Comparisons were made with respects to the value of the AL-28 rats, fixed as 1. *p < 0.05 versus the value of the AL-28 rats; n = number of analyzed animals.
Table 2.
Individual values of MFN2, DRP1 and Fusion index.
| MFN2/VDACa | DRP1/VDACa | Fusion index | |
|---|---|---|---|
| AL-28 | 0.21 | 0.33 | 0.63 |
| 0.44 | 0.18 | 2.44 | |
| 0.22 | 0.26 | 0.83 | |
| 0.36 | 0.27 | 1.31 | |
| 0.66 | 0.20 | 3.26 | |
| AL-32 | 0.77 | 0.56 | 1.36 |
| 0.46 | 0.52 | 0.88 | |
| 0.60 | 0.41 | 1.46 | |
| 1.27 | 0.66 | 1.92 | |
| 1.05 | 0.56 | 1.89 |
The values of MNF2 and DRP1 are expressed as relative amounts, determined by densitometry analysis. The densitometric value of OD units of every MFN2 or DRP1 band was related to the number of OD units of the respective band of VDAC for each analyzed sample.
Fig. 6.
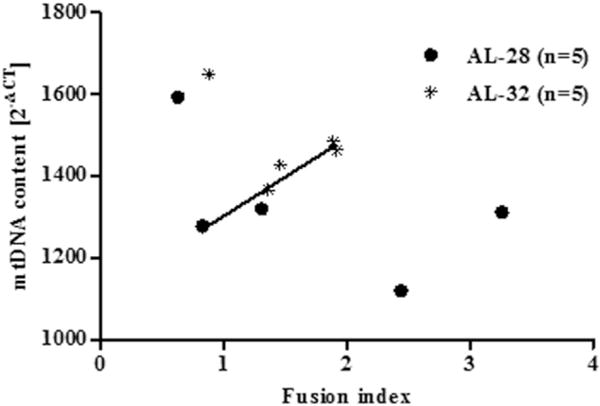
Correlation between the value of fusion index and the mtDNA content. A strong positive correlation was found between the fusion index value and the mtDNA content in four out of five animals from the AL-32 group and two from the AL-28 rats (see Table 2 for individual fusion indexes). The RT-PCR quantification of the mtDNA content in AL-28 and AL-32 rats, all normalized to β-actin, was performed according to the Pfaffl mathematical model and the values resulted from the calculation of the formula 2−ΔCT (Pfaffl 2001). Pearson’s test was performed and demonstrated a highly significant correlation (p < 0.005, correlation coefficient: 0.8931) in the AL-32 group (n = 4,) and AL-28 rats (n = 2) (filled line). The outlier from the AL-32 group and one animal from the AL-28 group shared the lowest fusion indexes together with the highest mtDNA contents, whereas the AL-28 rats with the highest fusion indexes were characterized by quite low mtDNA contents.
4. Discussion
Since a long time, the continuous growing interest for aging has focused also on those individuals, humans or animals, skipping the usual pace of the process and reaching older ages still with functions similar to those of younger counterparts. The puzzle of “the successful aging of centenarians” has originated several very relevant and updated studies that have unveiled part of a complicated story (Capri et al. 2014), dealing with various mechanisms eventually underlying a healthy longevity. In the search for clarifying such mechanisms also animal studies can become relevant. We had the possibility to examine liver samples from six old, AL-28, and six very old, AL-32, rats and we tried to find meaningful differences in the behavior of markers of mitochondrial functionality and biogenesis to get some hints about the longevity of the oldest animals. The studies comparing old and very old rats are in a limited number (Marzetti et al. 2008, Baker and Hepple, 2006, Hepple et al. 2004, Hagen et al. 2004) and this adds novelty to our data, although references will be made also to comparisons between young/adult and old/very old rats. The analysis of citrate synthase and Prx III proteins revealed the absence of a statistically significant change for both in the very old rats, indicative of an interruption in the already described, age-related decline in mitochondrial metabolism of rat liver (Cahill et al. 2005; Musicco et al. 2009; Navarro and Boveris 2004). In particular, the persistency of the protein amount of citrate synthase with extended aging might, however, be not controversial with the longevity-decreased activity of the same enzyme in rat plantaris muscle from 28–30 months to 36 months (Hagen et al. 2004). As for the Prx III protein, our previous results in rat liver showed an age-related reduced expression only passing from the middle age AL-18 animals to the AL-28 ones without ulterior decrease (Picca et al. 2013a). The present analysis, performed on a larger number of AL-32 animals, confirms such data with no longevity-related change in the protein amount. Similarly to the above quoted study (Picca et al. 2013a), we found no decrease in the mtDNA content in the older AL-32 rats with respects to the less aged ones. This suggests that the age-related loss of mtDNA, already reported in liver and other tissues of rats not older than 26–28 months (Picca et al. 2014, 2013a, 2013b), might have stopped or reduced its pace in the long-living AL-32 rats, reaching a sort of plateau level needed for life-compatibility. In support of this idea there was also the absence of a significant, longevity-related change in the amount of the mtDNA histone-like protein TFAM in the presently analyzed AL-32 animals that confirms our previous study as for the age-matched groups of rats (Picca et al. 2013a). Such lack of a longevity-related change in TFAM amount in the AL-32 rats might be due to the 50% reduced expression of the Lon protease, responsible for turn-over of exhausted TFAM (Lu et al. 2013; Matsushima et al. 2010). This is a particularly intriguing finding because previous reports in the literature demonstrated an age-related decrease in Lon activity and/or expression (Ngo et al. 2013) in rat liver (Bakala et al. 2003) and murine skeletal muscle (Bota et al. 2002) from animals up to 27 months of age. A possible explanation for such longevity-decreased expression of Lon might refer to its activity in the removal of TFAM unbound to mtDNA (Lu et al. 2013). We previously demonstrated a specific age-related, increased TFAM-binding to liver mtDNA regions encompassing the origins of replication in 28-month-old rats. Such results were related to the decrease in mtDNA content and TFAM amount found in the same animals, suggesting that the increased TFAM binding might have reduced mtDNA replication and repair as well as TFAM expression (Picca et al. 2013a). TFAM expression did not change between the AL-28 and the AL-32 rats and if the protein bound to the mitochondrial genome in the older animals in a way very similar to the AL-28 pattern, the amount of free TFAM, available for Lon degradation, in the AL-32 rats should have been quite limited. This might have induced the decreased expression of Lon that we described in the AL-32 animals and that might fit well in our model for longevity. Our hypothesis about the mitochondrial contribution to the natural longevity of the long-living AL-32 rats proposes that they were able to survive also because their mtDNA and related functional proteins had reached a “stationary-like” situation with a controlled molecular turnover through balance of damage, repair, elimination and new synthesis. Such hypothesis would explain also the absence of longevity-related changes in the expression of two major enzymes active in the BER process of mtDNA namely OGG1 and APE1. The unchanged amounts of OGG1 and APE1 inside mitochondria suggested that BER in the AL-32 rats was still likely active as in the AL-28 animals and should have thus prevented an increased loss of mtDNA, helping to maintain the “stationary”, life-compatible mtDNA content. Effectively, previous reports showed an age-increased OGG1 activity in rat liver and mouse liver mitochondrial extracts up to only 23 months of age (De Souza-Pinto et al. 2001; Souza-Pinto et al. 1999), although the protein amount was not determined. More recently, the single-nucleotide-BER, the APE1 and the long-patch-BER activities were measured in five tissues from aged mouse, demonstrating an age-related increase in the mitochondrial activities of liver, again only up to 20 months of age and without a determination of the proteins amount (Szczesny et al. 2010). The absence of statistically significant, longevity-related changes in the mitochondrial OGG1 and APE1 proteins amounts here presented supports, consistently with the reported age-increased activity of the two repair enzymes, the persistency of repair mechanisms in the AL-32 rats. The maintenance of mtDNA content and likely also integrity in the AL-32 rats also suggests that the initiation of the mitochondrial intrinsic apoptotic pathway, which should be related to the accumulation of DNA damage (Green and Llambi 2015), might have not increased in the older animals with respects to what occurred in the AL-28 counterparts. The determination of the intra-mitochondrial amount of cytochrome c was a reliable evaluation of the protein release from the intermembrane space into the cytosol and of the sequential activation of the caspase-dependent apoptotic cascade. The 50% increased amount of cytochrome c inside mitochondria in the AL-32 rats with respects to the AL-28 counterparts strongly supports a reduced initiation of the intrinsic apoptotic pathway in the older animals. To reinforce this indication we found in the AL-32 rats an unchanged cytosolic amount of cytochrome c (results not shown) that paralleled the results by (Marzetti et al. 2008). Such Authors suggested a marginal involvement of the mitochondrial caspase-dependent apoptotic pathway in the age-associated muscle wasting of senescent (37-month-old) rats and this might have occurred also in liver from AL-32 rats. It appears that the “stationary” situation, established in the AL-32 animals, would have obtained an efficient turnover of dysfunctional mitochondria and cells, also through the age-related altered regulation of intrinsic apoptosis reported in 25-month-old rat liver (Mach et al. 2015). Of course, having hypothesized that an equilibrium between survival and removal had been reached inside mitochondria and cells from the AL-32 rats, we analyzed two proteins crucial in the fine-tuned balance of mitochondrial dynamics namely MFN2 and DRP1. The finding of doubled mean amounts for both proteins in the AL-32 group compared to the AL-28 mean values was unexpected. In facts, aging effects, reported in literature, implied a dysregulation of dynamics balance (Gaziev et al. 2014) and a marked reduction in fission, leading to a diminished removal of damaged organelles through fission (Mai et al. 2010). In the latter study about senescent human endothelial cells it was shown that the age-reduced expression of Fis1 and DRP1 mediated mitochondrial elongation (namely morphological evidence of increased fusion) and enhanced resistance to oxidative stress (Mai et al. 2010). The indication of an age-related prevalence of fusion above fission was confirmed by (Leduc-Gaudet et al. 2015) who reported an increased fusion index in aged (22–24-month-old) mice skeletal muscle. They also suggested the association between increased fusion index and sarcopenia because of insufficient removal of damaged organelles due to reduced fission (Leduc-Gaudet et al. 2015). We analyzed the specific MFN2 and DRP1 amounts and calculated the fusion index in all assayed animals (Table 2), finding that such indexes in the AL-28 rats were very different, ranging from 0.63 through 3.26 with two animals presenting the highest values of MFN2 amount and fusion index. On the contrary, all the AL-32 fusion indexes grouped between 0.88 and 1.92, with the DRP1 amount generally less far from the MFN2 counterpart than in the AL-28 rats. This suggests the existence of a “threshold” fusion index around the value of 2.0, not exceeded in long-living rats and likely related to their longevity. In our model, below such threshold, fission was still able to efficiently remove damaged organelles and fusion could ensure a communication among mitochondria able to diffuse damaged macromolecules in the mitochondrial network and smooth their effects. Previous results from 28-month-old rat liver, indicating the age-related decrease in mtDNA content as well as in DRP1 and MFN2 amounts (Pesce et al. 2012), prompted us to search a relationship between these parameters in our examined animals. In fact, our hypothesis was further supported by the novel analysis, through linear regression, of fusion index and mtDNA content in all assayed rats, demonstrating a significant positive correlation between the two variables for two AL-28 rats and almost all AL-32 animals. The two AL-28 animals characterized by the highest fusion indexes (2.44 and 3.26) did not share such correlation and harbored low mtDNA contents as if fusion strongly prevailing above fission was leading to an accumulation of damaged macromolecules, as suggested also by (Scheibye-Knudsen et al. 2015), and to a reduction in the mtDNA content. The values of the fusion index, demonstrating the strong positive correlation with the respective mtDNA contents, grouped all below 2.0, indicating that a limited prevalence of fusion above a still active fission might have ensured a functional mitochondrial network, able to perform communication as well as removal of damaged parts and organelles. Such finely tuned-up balance of mitochondrial dynamics should have led to a quite narrow range of high mtDNA contents, likely the best-suitable for longevity, through modulation of the activities involved in mtDNA maintenance and turnover. The AL-32 outlier and one rat from the AL-28 group, featuring, respectively, 0.88 and 0.63 as fusion indexes together with the highest mtDNA contents (1649.09 and 1593.18) might indicate that when fission was more active than fusion the maintenance of mtDNA was extremely well-guaranteed and, probably, the overall functionality of mitochondria still kept a sort of “adult-like performance”, favoring longevity. Our results support the idea that the persistency of adult-like dynamics in the AL-32 rats might positively affect also mtDNA integrity through enzymes for mtDNA repair as well as the expression of TFAM and Lon, adapting mtDNA content and functions to the always changing cell demands. Recent reports have demonstrated that a number of phenomena are regulated through the balance of mitochondrial dynamics (Gillies and Kuwana 2014) and such balance can be modulated by nutrient status and metabolic alterations (Wai and Langer 2016). In particular, MFN2 has been reported to be regulated by the p53 tumor suppressor (Wang et al. 2010), which can be activated by various stresses including DNA damage, oxidative and nutritional stress and ischemia and thus might influence mitochondrial dynamics. By the other side, the effects of activated p53 on mitochondria can be elicited also along molecular pathways not targeting the organelle dynamics. A mild metabolic stress, as fasting or the chemically-induced oxidative unbalance due to GSH depletion, has been shown to take to a p53-mediated peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) up-regulation that induced expression of the mitochondrial antioxidant superoxide dismutase (SOD2) and reinforced the organelle’s response to oxidative stress (Aquilano et al. 2013). Actions on mitochondria orchestrated by p53 include also mitophagy, crucial for the cellular redox homeostasis and modulated by a growing number of proteins that reside in the mitochondria or become associated with mitochondria for their function, like PINK1 and parkin (Wang et al. 2014). It has been recently shown that mitochondria from skeletal muscle of p53 KO mice display alterations in mitophagy-related as well as fission and fusion proteins (Saleem et al. 2015), highlighting the interconnections among all pathways involved in mitochondrial quality control. An indirect confirmation about the role of interconnected mitochondrial quality control pathways derives from the reduced mitophagy, reported in accelerated aging disorders and due to persisting mtDNA damage, that prevented mitochondrial biogenesis and led to dysfunctional organelles (Fang et al. 2014). Therefore, the achievement of an adult-like “stationary” situation of balanced mitochondrial dynamics can affect various mitochondrial processes and reasonably contribute to a successful and long aging, as inferred from our results. More studies are needed along this new pathway to shed light on the causes and the effects of the age-related unbalance of mitochondrial dynamics and mtDNA content and their relevance for natural longevity.
5. Conclusions
The present data show that liver from the naturally long-living AL-32 rats is mainly characterized, with respects to that from the less aged AL-28 counterparts, by a doubling of both proteins MFN2 and DRP1, very relevant for mitochondrial dynamics, and a narrow range of fusion index values. Furthermore, most AL-32 rats and some AL-28 ones demonstrate a strong correlation between the respective fusion indexes and mtDNA contents, indicating that a limited prevalence of fusion over fission correlates with a high mtDNA content and their coordination likely favors a better life-compatibility. We hypothesize that the mitochondrial contribution to extended individual longevity might imply the establishment of an adult-like “stationary” situation, reached through an optimal balance of interconnected different quality control pathways, as those here assayed of mitochondrial dynamics and regulation of mtDNA content. The relevance of the issues requires further study to proceed in the elucidation of the involved mechanisms.
Acknowledgments
This research was supported by grants to AMSL (University of Bari-Progetti di Ateneo 2012; Istituto Banco di Napoli-Fondazione 2015) and to CL (National Institute on Aging (NIA) R01 AG17994). We greatly thank Dr Carolyn K. Suzuki, Department of Biochemistry and Molecular Biology, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, for the generous gift of the antibody against Lon and for helpful suggestions with the work. We gratefully acknowledge Dr. Antonella Bobba, IBBE-CNR Bari (Italy), for the kind gift of the antibody against Cyt c.
Footnotes
Conflict of interest
The authors have no actual or potential conflict of interest associated with this research.
References
- Aquilano K, Baldelli S, Pagliei B, Cannata SM, Rotilio G, Ciriolo MR. p53 orchestrates the PGC-1α-mediated antioxidant response upon mild redox and meta-bolic imbalance. Antioxid Redox Signal. 2013;18:386–399. doi: 10.1089/ars.2012.4615. http://dx.doi.org/10.1089/ars.2012.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakala H, Delaval E, Hamelin M, Bismuth J, Borot-Laloi C, Corman B, Friguet B. Changes in rat liver mitochondria with aging. Lon protease-like reactivity and N(epsilon)-carboxymethyllysine accumulation in the matrix. Eur J Biochem. 2003;270:2295–2302. doi: 10.1046/j.1432-1033.2003.03598.x. http://dx.doi.org/10.1046/j.1432-1033.2003.03598.x. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Hepple RT. Elevated caspase and AIF gene expression correlate with progression of sarcopenia during aging in male F344BN rats. Exp Gerontol. 2006;41:1149–1156. doi: 10.1016/j.exger.2006.08.007. http://dx.doi.org/10.1016/j.exger.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Betik AC, Thomas MM, Wright KJ, Riel CD, Hepple RT. Exercise training from late middle age until senescence does not attenuate the declines in skeletal muscle aerobic function. Am J Phys Regul Integr Comp Phys. 2009;297:R744–R755. doi: 10.1152/ajpregu.90959.2008. http://dx.doi.org/10.1152/ajpregu.90959.2008. [DOI] [PubMed] [Google Scholar]
- Bota DA, Van Remmen H, Davies KJA. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002;532:103–106. doi: 10.1016/s0014-5793(02)03638-4. http://dx.doi.org/10.1016/S0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- Cahill A, Hershman S, Davies A, Sykora P. Ethanol feeding enhances age-related deterioration of the rat hepatic mitochondrion. Am J Physiol Gastrointest Liver Physiol. 2005;289:1115–1123. doi: 10.1152/ajpgi.00193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capri M, Santoro A, Garagnani P, Mg B, Olivieri PC, Salvioli PA, Franceschi C. Genes of human longevity: an endless quest? Curr Vasc Pharmacol. 2014;12:707–717. doi: 10.2174/1570161111666131219110301. [DOI] [PubMed] [Google Scholar]
- Chang TS, Cho CS, Park S, Yu S, Sang WK, Sue GR. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. http://dx.doi.org/10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- De Souza-Pinto NC, Hogue BA, Bohr VA. DNA repair and aging in mouse liver: 8-oxodG glycosylase activity increase in mitochondrial but not in nuclear extracts. Free Radic Biol Med. 2001;30:916–923. doi: 10.1016/s0891-5849(01)00483-x. http://dx.doi.org/10.1016/S0891-5849(01)00483-X. [DOI] [PubMed] [Google Scholar]
- Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, Mitchell JR, Croteau DL, Bohr VA. Defective mitophagy in XPA via PARP1 hyperactivation and NAD +/SIRT1 reduction. Cell. 2014;157:882–896. doi: 10.1016/j.cell.2014.03.026. http://dx.doi.org/10.1016/j.cell.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziev AI, Abdullaev S, Podlutsky A. Mitochondrial function and mitochondrial DNA maintenance with advancing age. Biogerontology. 2014;15:417–438. doi: 10.1007/s10522-014-9515-2. http://dx.doi.org/10.1007/s10522-014-9515-2. [DOI] [PubMed] [Google Scholar]
- Gillies LA, Kuwana T. Apoptosis regulation at the mitochondrial outer membrane. J Cell Biochem. 2014;115:632–640. doi: 10.1002/jcb.24709. http://dx.doi.org/10.1002/jcb.24709. [DOI] [PubMed] [Google Scholar]
- Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015:7. doi: 10.1101/cshperspect.a006080. http://dx.doi.org/10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed]
- Hagen JL, Krause DJ, Baker DJ, Fu MH, Tarnopolsky MA, Hepple RT, Al HET. Skeletal muscle aging in F344BN F1-hybrid rats: I. Mitochondrial dysfunction contributes to the age-associated reduction in VO2max. J Gerontol A Biol Sci Med Sci. 2004;59A:1099–1110. doi: 10.1093/gerona/59.11.1099. [DOI] [PubMed] [Google Scholar]
- Held NM, Houtkooper RH. Mitochondrial quality control pathways as determinants of metabolic health. BioEssays. 2015;37:867–876. doi: 10.1002/bies.201500013. http://dx.doi.org/10.1002/bies.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT, Hagen JL, Krause DJ, Baker DJ. Skeletal muscle aging in F344BN F1-hybrid rats: II. Improved contractile economy in senescence helps compensate for reduced ATP-generating capacity. J Gerontol A Biol Sci Med Sci. 2004;59:1111–1119. doi: 10.1093/gerona/59.11.1111. [DOI] [PubMed] [Google Scholar]
- Hu F, Liu F. Targeting tissue-specific metabolic signaling pathways in aging: the promise and limitations. Protein Cell. 2014;5:21–35. doi: 10.1007/s13238-013-0002-3. http://dx.doi.org/10.1007/s13238-013-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Chung E, Diffee G, Ji LL. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1?? Exp Gerontol. 2013;48:1343–1350. doi: 10.1016/j.exger.2013.08.004. http://dx.doi.org/10.1016/j.exger.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014;24:761–770. doi: 10.1016/j.tcb.2014.08.005. http://dx.doi.org/10.1016/j.tcb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Leduc-Gaudet JP, Picard M, St-Jean Pelletier F, Sgarioto N, Auger MJ, Vallée J, Robitaille R, St-Pierre DH, Gouspillou G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 17923 Oncotarget. 2015;6:17923–17937. doi: 10.18632/oncotarget.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Jin Shin Y, Chun Cho S, Lee SM, Jae Bahn Y, Young Kim J, Kwon ES, Chul Park S, Goo Rhee S, Ae Woo H, Kwon KS. Peroxiredoxin 3 has a crucial role in the contractile function of skeletal muscle via regulating mitochondrial homeostasis. Free Radic Biol Med. 2014;77:1–9. doi: 10.1016/j.freeradbiomed.2014.09.010. http://dx.doi.org/10.1016/j.freeradbiomed.2014.09.010. [DOI] [PubMed] [Google Scholar]
- López-Lluch G, Santos-Ocaña C, Sánchez-Alcázar JA, Fernández-Ayala DJM, Asencio-Salcedo C, Rodríguez-Aguilera JC, Navas P. Mitochondrial responsibility in ageing process: innocent, suspect or guilty. Biogerontology. 2015:599–620. doi: 10.1007/s10522-015-9585-9. http://dx.doi.org/10.1007/s10522-015-9585-9. [DOI] [PubMed]
- Lu B, Lee J, Nie X, Li M, Morozov YI, Venkatesh S, Bogenhagen DF, Temiakov D, Suzuki CK. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol Cell. 2013;49:121–132. doi: 10.1016/j.molcel.2012.10.023. http://dx.doi.org/10.1016/j.molcel.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach J, Huizer-Pajkos A, Kane A, Jones B, McKenzie C, Mitchell SJ, de Cabo R, Cogger VC, Le Couteur DG, Hilmer SN. The effect of aging on mitochondrial and cytosolic hepatic intrinsic death pathway and apoptosis associated proteins in Fischer 344 rats. Exp Gerontol. 2015;67:54–61. doi: 10.1016/j.exger.2015.04.009. http://dx.doi.org/10.1016/j.exger.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai S, Klinkenberg M, Auburger G, Bereiter-Hahn J, Jendrach M. Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J Cell Sci. 2010;123:917–926. doi: 10.1242/jcs.059246. http://dx.doi.org/10.1242/jcs.059246. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Eva S, Anne H, Chung H, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008;129:542–549. doi: 10.1016/j.mad.2008.05.005. http://dx.doi.org/10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima Y, Goto Y, Kaguni LS. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM) Proc Natl Acad Sci U S A. 2010;107:18410–18415. doi: 10.1073/pnas.1008924107. http://dx.doi.org/10.1073/pnas.1008924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicco C, Capelli V, Pesce V, Timperio AM, Calvani M, Mosconi L, Zolla L, Cantatore P, Gadaleta MN. Accumulation of overoxidized Peroxiredoxin III in aged rat liver mitochondria. Biochim Biophys Acta Bioenerg. 2009;1787:890–896. doi: 10.1016/j.bbabio.2009.03.002. http://dx.doi.org/10.1016/j.bbabio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Phys Regul Integr Comp Phys. 2004;287:R1244–R1249. doi: 10.1152/ajpregu.00226.2004. http://dx.doi.org/10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- Ngo JK, Pomatto LCD, Davies KJA. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging$ Redox Biol. 2013;1:258–264. doi: 10.1016/j.redox.2013.01.015. http://dx.doi.org/10.1016/j.redox.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce V, Cormio A, Fracasso F, Lezza AMS, Cantatore P, Gadaleta MN. Age-related changes of mitochondrial DNA content and mitochondrial genotypic and phenotypic alterations in rat hind-limb skeletal muscles. J Gerontol A Biol Sci Med Sci. 2005;60:715–723. doi: 10.1093/gerona/60.6.715. [DOI] [PubMed] [Google Scholar]
- Pesce V, Nicassio L, Fracasso F, Musicco C, Cantatore P, Gadaleta MN. Acetyl-L-Carnitine activates the Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α/PGC-1β-dependent signaling cascade of mitochondrial biogenesis and decreases the oxidized peroxyredoxins content in old rat liver. Rejuvenation Res. 2012;15:136–139. doi: 10.1089/rej.2011.1255. http://dx.doi.org/10.1089/rej.2011.1255. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. http://dx.doi.org/10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picca A, Lezza AMS. Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions. Useful insights from aging and calorie restriction studies. Mitochondrion. 2015;25:67–75. doi: 10.1016/j.mito.2015.10.001. http://dx.doi.org/10.1016/j.mito.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Picca A, Fracasso F, Pesce V, Cantatore P, Joseph AM, Leeuwenburgh C, Gadaleta MN, Lezza AMS. Age- and calorie restriction-related changes in rat brain mitochondrial DNA and TFAM binding. Age (Omaha) 2013a;35:1607–1620. doi: 10.1007/s11357-012-9465-z. http://dx.doi.org/10.1007/s11357-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picca A, Pesce V, Fracasso F, Joseph AM, Leeuwenburgh C, Lezza AMS. Aging and calorie restriction oppositely affect mitochondrial biogenesis through TFAM binding at both origins of mitochondrial DNA replication in rat liver. PLoS One. 2013b;8:1–14. doi: 10.1371/journal.pone.0074644. http://dx.doi.org/10.1371/journal.pone.0074644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picca A, Pesce V, Fracasso F, Joseph AM, Leeuwenburgh C, Lezza AMS. A comparison among the tissue-specific effects of aging and calorie restriction on TFAM amount and TFAM-binding activity to mtDNA in rat. Biochim Biophys Acta, Gen Subj. 2014;1840:2184–2191. doi: 10.1016/j.bbagen.2014.03.004. http://dx.doi.org/10.1016/j.bbagen.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem A, Iqbal S, Zhang Y, Hood DA. Effect of p53 on mitochondrial morphology, import and assembly in skeletal muscle. Am J Phys Cell Physiol. 2015;308:C319–C329. doi: 10.1152/ajpcell.00253.2014. http://dx.doi.org/10.1152/ajpcell.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Fang EF, Croteau DL, Wilson DM, Bohr VA. Protecting the mitochondrial powerhouse. Trends Cell Biol. 2015;25:158–170. doi: 10.1016/j.tcb.2014.11.002. http://dx.doi.org/10.1016/j.tcb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L. Opening the doors to cytochrome c: changes in mitochondrial shape and apoptosis. Int J Biochem Cell Biol. 2009;41:1875–1883. doi: 10.1016/j.biocel.2009.04.016. http://dx.doi.org/10.1016/j.biocel.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Souza-Pinto NC, Croteau DL, Hudson EK, Hansford RG, Bohr VA. Age-associated increase in 8-oxo-deoxyguanosine glycosylase/AP lyase activity in rat mitochondria. Nucleic Acids Res. 1999;27:1935–1942. doi: 10.1093/nar/27.8.1935. http://dx.doi.org/10.1093/nar/27.8.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny B, Tann AW, Mitra S. Age- and tissue-specific changes in mitochondrial and nuclear DNA base excision repair activity in mice: susceptibility of skeletal muscles to oxidative injury. Mech Ageing Dev. 2010;131:330–337. doi: 10.1016/j.mad.2010.03.009. http://dx.doi.org/10.1016/j.mad.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. http://dx.doi.org/10.1093/gerona/54.11.B492. [DOI] [PubMed] [Google Scholar]
- Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;xx:1–13. doi: 10.1016/j.tem.2015.12.001. http://dx.doi.org/10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Wang W, Cheng X, Lu J, Wei J, Fu G, Zhu F, Jia C, Zhou L, Xie H, Zheng S. Mitofusin-2 is a novel direct target of p53. Biochem Biophys Res Commun. 2010;400:587–592. doi: 10.1016/j.bbrc.2010.08.108. http://dx.doi.org/10.1016/j.bbrc.2010.08.108. [DOI] [PubMed] [Google Scholar]
- Wang DB, Kinoshita C, Kinoshita Y, Morrison RS. P53 and mitochondrial function in neurons. Biochim Biophys Acta Mol basis Dis. 2014;1842:1186–1197. doi: 10.1016/j.bbadis.2013.12.015. http://dx.doi.org/10.1016/j.bbadis.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wredenberg A, Wibom R, Wilhelmsson H, Graff C, Wiener HH, Burden SJ, Oldfors A, Westerblad H, Larsson NG. Increased mitochondrial mass in mitochondrial myopathy mice. Proc Natl Acad Sci U S A. 2002;99:15066–15071. doi: 10.1073/pnas.232591499. http://dx.doi.org/10.1073/pnas.232591499. [DOI] [PMC free article] [PubMed] [Google Scholar]


