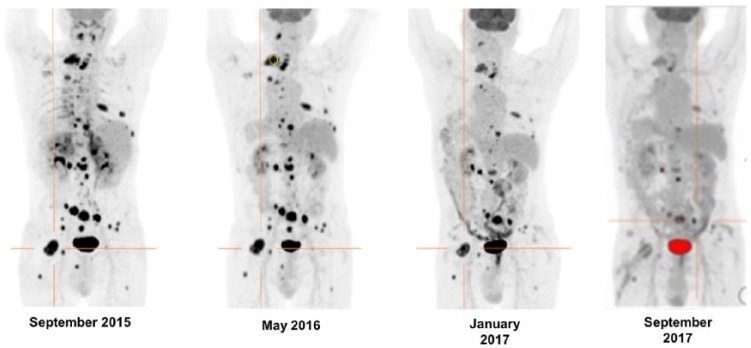
Figure 1.
This image demonstrates the metabolic changes observed on the 18F-FDG PET/CT at baseline (prior to initiating MS with TMZ in September 2015), restaging studies performed after 5 cycles, in May 2016, and 15 cycles, in March 2017, of the chemotherapy regimen consisting of MS with TMZ and high-dose lanreotide. In September 2015, maximum standardized uptake value (SUVmax) normalized to lean body mass (SULmax) of the most active lesion (marked in orange) was 23.12. In May 2016, a PMR was noted (SULmax) of the target lesion = 12.49, which is 45.9% reduction). In March and September 2017, there was stabilization of the PMR and no new lesions were detected on either of the studies. 18F-FDG PET/CT: 18F-fluorodeoxyglucose positron emission tomography/computed tomography; PMR: partial metabolic response; TMZ: temozolomide.