Figure 1. Zebrafish zeppelin (zep) mutant embryos exhibit edema, a decrease in kidney podocyte (P) cells, and an increased interrenal gland (IR).
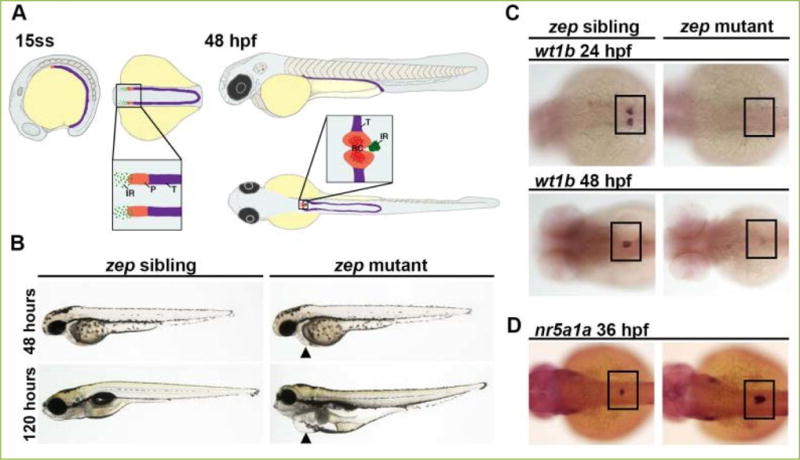
A) The zebrafish kidney arises from the intermediate mesoderm (IM, purple) and by the 15 somite stage (ss) the anteriormost region of the IM is patterned by two fields of P progenitors, as distinguished by the marker wt1a, whereas non-wt1a expressing cells are predestined as kidney tubule (T). It is hypothesized that IR precursors arise from the wt1a-expressing portion of IM. The P progenitor fields begin to migrate towards the midline after 24 hpf, and by 48 hpf this field is fused at the midline, has become innervated with capillaries, and begins to function as the renal corpuscle (RC). The IR cells also exhibit a migration toward the midline and fuse to become a single organ by the 36 hpf time point. B) Brightfield imaging showing live morphology: zep mutants appeared similar to siblings at the 48 hpf time point, but by 120 hpf they exhibited severe edema including around the pericardial region (black arrowheads). C) zep mutants exhibited a severe decrease in podocytes at 24 hpf and 48 hpf compared to siblings, demonstrated by whole mount in situ hybridization (WISH) using the podocyte marker wt1b. D) WISH on zep mutants at 36 hpf with the IR marker nr5a1a showed an increased IR in mutants compared to siblings. C–D) Black boxes demarcate the embryonic region where P or IR areas are located. Figure adapted from[66], and reprinted as allowed by the terms of the CC BY 4.0 Creative Commons License of the Authors.
