Figure 6.
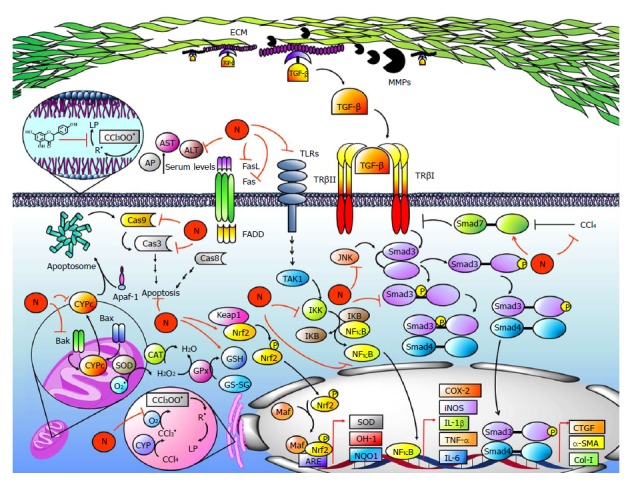
Naringenin prevents acute and chronic CCl4-induced liver damage. Carbon tetrachloride (CCl4) is activated by CYP2E1, CYP2B1, CYP2B2, and CYP3A (CYP) to form the trichloromethyl radical (CCl3•); then, it reacts with the oxygen-forming trichloromethylperoxy radical (CCl3OO•). The CCl3OO• initiates lipid peroxidation (LP), free radical (R•) generation, and imbalance of the endogenous antioxidant system formed by superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione (GSH), heme oxygenase (OH-1), NADPH quinone oxidoreductase (NQO1) and nuclear factor-erythroid 2 related factor 2 (Nrf2). Naringenin prevents CCl4 metabolism, LP and imbalance of the antioxidant system. Naringenin also prevents increased levels of alkaline phosphatase (AP), aspartate aminotransferase (AST), alanine aminotransaminase (ALT), and γ-glutamyl transferase (GGT). On the other hand, CCl4 increases intrinsic and extrinsic apoptosis pathways in hepatocytes; however, naringenin prevents CYPc release, as well as BCL2-associated X protein (Bax), BCL2-antagonist/killer 1 (Bak), Caspase 3 (Cas3) and Caspase 9 (Cas9) elevation, a protein related with the intrinsic pathway. For the extrinsic apoptosis pathway, naringenin prevents Fas and Fas ligand (FasL) increases produced by CCl4 administration. During CCl4-induced fibrosis, there is a proinflammatory environment generated by Kupffer cells and HSCs. The NF-κB pathway starts when TLRs are activated; then, intermediates are activated leading to inhibitor κB (IKB) phosphorylation by IκB kinase (IKK) and NF-κB release. NF-κB regulates inflammatory protein expression, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), cyclooxygenase-2 (COX-2), interleukin-1 (IL-1) and inducible nitric oxide synthase (iNOS), while naringenin maintains normal levels of these proteins during CCl4-induced liver damage. Transforming growth factor-β (TGF-β) activates receptor-activated Smad3 (Smad3), leading to transcriptional induction of α-smooth muscle actin (α-SMA), connective tissue growth factor (CTGF), and collagen-1 (Col-1). Moreover, Smad3 is also activated by JNK via linker domain phosphorylation. Naringenin prevents Smad3 activation and α-SMA, CTGF, and Col-1 elevation because it inhibits TGF-β elevation and JNK activation. Metalloproteases (MMPs) cleave extra cellular matrix (ECM) proteins, favoring TGF-β release as well as HSC migration to other sites, increasing fibrosis development; naringenin prevents MMPs elevation. On the other hand, CCl4 decreases Smad7 protein levels; this protein inhibits the TGF-β signaling pathway by TGF-β receptor I (TβRI) ubiquitination, but nevertheless, naringenin maintains normal levels of Smad7 during CCl4 treatment.
