Figure 8.
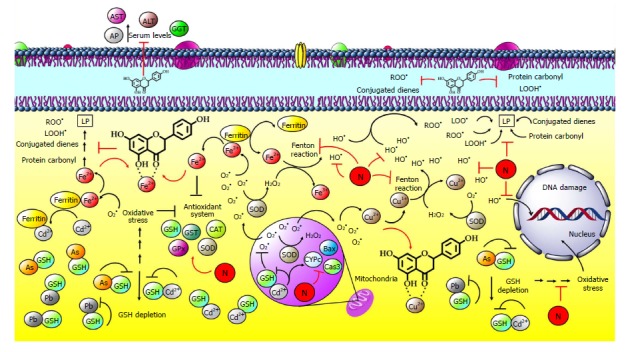
Naringenin inhibits hepatic damage induced by heavy metals. Heavy metals can be classified according to the mechanism of action in redox-active metals such as iron (Fe) and copper (Cu) or redox-inactive metals such as cadmium (Cd), arsenic (As) and lead (Pb). One of the main free radicals is superoxide radical (O2•); normally, it is produced by NADP oxidase, and then, it is neutralized by superoxide dismutase (SOD), generating hydrogen peroxide (H2O2). Intracellular Fe is releases from ferritin by O2•; next, free Fe reacts with H2O2 in the Fenton reaction, generating high amounts of hydroxyl radicals (OH•). After that, OH• attacks double bonds of DNA bases. In the case of lipids, free Fe produces lipid peroxidation (LP) through peroxyl radicals (ROO•), producing lipid hydroperoxides (LOOH•), conjugated dienes and protein carbonyl. Regarding Cu, once inside the hepatocyte, Cu ion (Cu2+), can be reduced to cuprous ion (Cu1+) when reacting with O2•; then, it mediates H2O2 decomposition in OH• via the Fenton reaction. These processes result in hepatocyte and liver damage. Naringenin can chelate these metals, preventing their participation in the Fenton reaction; naringenin also inhibits oxidative stress by its antioxidant capacity and by promoting the endogenous antioxidant system. On the other hand, redox-inactive metals such as Cd, arsenic (As) and lead (Pb) form complexes with thiol groups, such as glutathione (GSH), in the cytoplasm and mitochondria. GSH level reduction, GSH inactivation, and GSH system deregulation increase metal toxicity. In addition, Cd can replace Fe and Cu in ferritin or apoferritin; thus, free Fe and Cu ions cause oxidative stress via the Fenton reactions and elevation of BCL2-associated X protein (Bax), Caspase 3 (Cas3) and cytochrome (CYPc) proapoptotic proteins. Naringenin improves the antioxidant system by increasing SOD, catalase (CAT), glutathione peroxidase (GPx), glutathione transferase (GST) enzymes and GSH levels.
