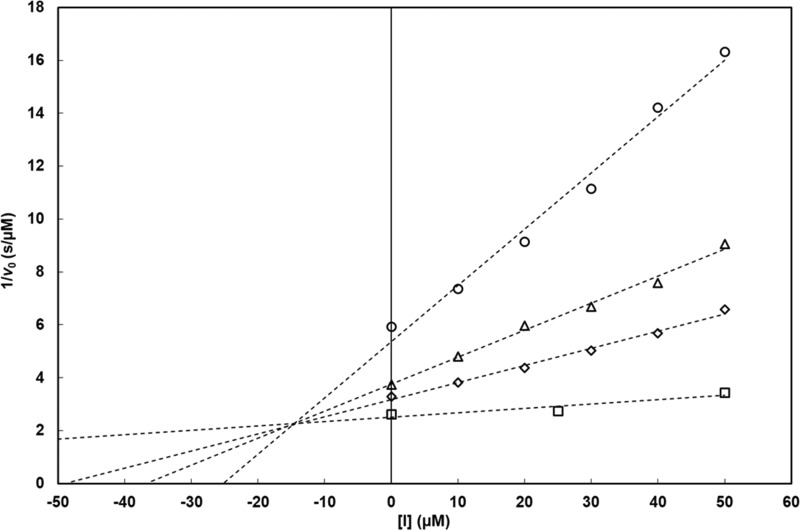
FIG 2.
Dixon analysis of the inhibition of VIM-1 by ANT431. Initial rates of β-lactam hydrolysis were measured spectrophotometrically using MEM (○, 40 μM; △, 90 μM; ◇, 130 μM; □, 800 μM) as the substrate in 50 mM HEPES buffer (pH 7.5), in the presence of 6.9 nM purified VIM-1. Inhibitor concentrations ranged from 10 to 50 μM. Initial rates were measured in triplicates (SD, ≤5%). Vmax was unaffected by ANT431. These data fully support a competitive mode of inhibition of the enzyme by ANT431, with a Ki value of 14.6 ± 0.6 μM. Similar conclusions (data not shown) were obtained with the NDM-1, IMP-1, and VIM-2 MBLs.