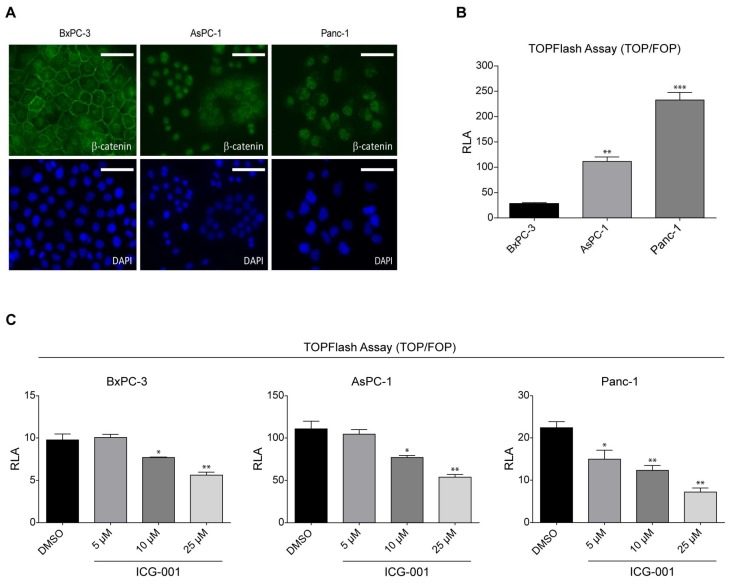
Figure 1.
K-Ras activating mutation increases Wnt/β-catenin signaling in human pancreatic cancer cells. (A) BxPC-3, AsPC-1, and PANC-1 pancreatic cancer cells were assayed by immunofluorescence to determine cellular localization of β-catenin. BxPC-3 cells, expressing wild type K-Ras, showed distinct membrane localization of β-catenin and weak, diffuse nuclear and cytoplasmic staining (DAPI, counterstain). However, in K-Ras mutant cells AsPC-1 and PANC-1 there was virtually no membrane-associated β-catenin. AsPC-1 cells showed diffuse cytoplasmic staining and strong nuclear staining for β-catenin, whereas PANC-1 cells demonstrated strong nuclear staining with limited cytoplasmic staining. Scale bar = 100 µm. (B) TOPFlash reporter gene assay was used to evaluate Wnt/β-catenin transcriptional activation in BxPC-3, AsPC-1, and PANC-1 cells. BxPC-3 cells exhibited weak Wnt/TCF/β-catenin-driven luciferase expression. However, AsPC-1 and PANC-1 cells demonstrated enhanced TOPFlash activity. n = 3, ** p < 0.01, *** p < 0.001, compared to BxPC-3. RLA, relative luciferase activity. (C) Treatment with ICG-001, a specific, small molecule inhibitor of CREB-binding protein (CBP)/β-catenin interaction, demonstrated dose-dependent decrease in TOPFlash activity in BxPC-3, AsPC-1, and PANC-1 cells. n = 3, * p < 0.05, ** p < 0.01, compared to DMSO control. RLA, relative luciferase activity.