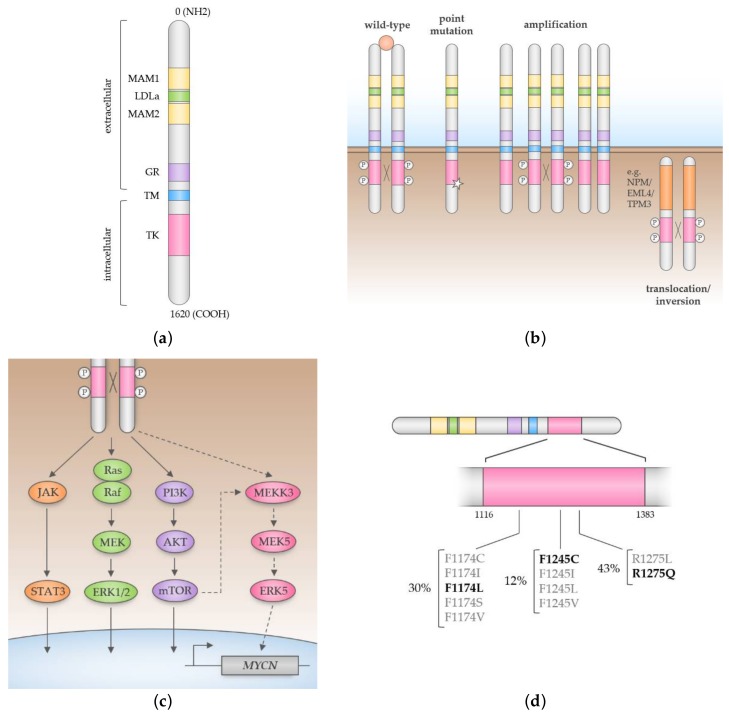
Figure 1.
(a) Domain structure of Anaplastic Lymphoma Kinase (ALK). The N-terminal extracellular domain comprises two MAM domains flanked by a low-density lipoprotein class A (LDLa) domain, and a glycine-rich (GR) domain. The C-terminal intracellular region comprises the tyrosine kinase (TK) domain; (b) In the wild-type receptor, ligand-induced dimerisation of the extracellular region permits auto- and transphosphorylation of the kinase domain and subsequent recruitment of signal transducers. Aberrant forms of ALK expressed in cancer are ligand-independent and are caused by point mutations in the kinase domain, gene amplification, or gene fusion; (c) Full-length ALK signals through the Ras/MAPK, PI3K/AKT and JAK/STAT pathways. In neuroblastoma, MYCN expression is activated in a pathway mediated by ALK, PI3K/AKT, MEKK3, MEK5 and ERK5 (dashed lines); (d) In neuroblastoma, gain-of-function mutations cluster in the kinase domain of ALK. Mutations in three key positions—F1174, F1245, and R1275—account for around 85% of ALK mutations in neuroblastoma. The wild-type forms of these residues maintain the kinase in an auto-inhibited conformation. The diagram shows (in bold) the most common mutation at each position.