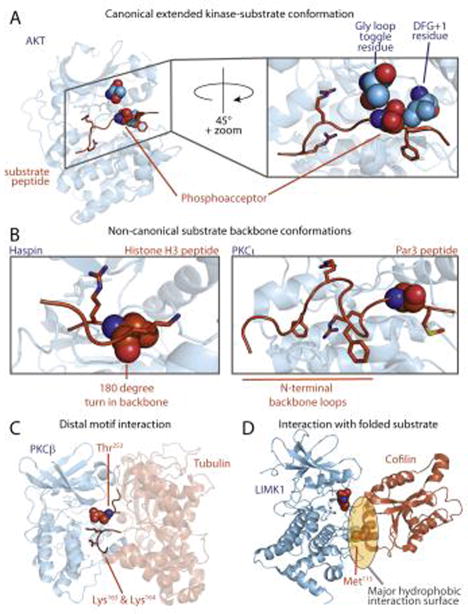
Figure 2.
Canonical and non-canonical kinase-substrate interactions. (A) Canonical peptide binding mode (PDB:1O6K). Substrate peptide binds in an extended conformation, with amino acid side-chains accessing distinct pockets. Kinase residues that determine phosphoacceptor residue specificity are shown in space fill, including the phosphorylatable residue in the Gly loop [8] (green) and the DFG+1 residue [7] in the activation loop (purple). (B) Atypical interactions between the kinase catalytic cleft and peptide substrates. The Haspin-histone H3 peptide complex, in which the peptide backbone makes a 180° turn near the phosphoacceptor, is shown at left (PDB: 4OUC). In the PKCι-Par3 peptide complex shown at right (PDB: 4DC2) the peptide backbone makes two β-turns N-terminal to the phosphoacceptor residue. (C) A model for PKCβ bound to tubulin, in which basic residues in the substrate distal in primary sequence are close to the phosphoacceptor residue in the 3D structure. (D) LIMK interacts with cofilin through a hydrophobic interaction centered on the αG helix of the kinase, in which placement of the phosphoacceptor requires a structured substrate (PDB: 5HVK). The Met115 residue on cofilin is critical for LIMK recognition.