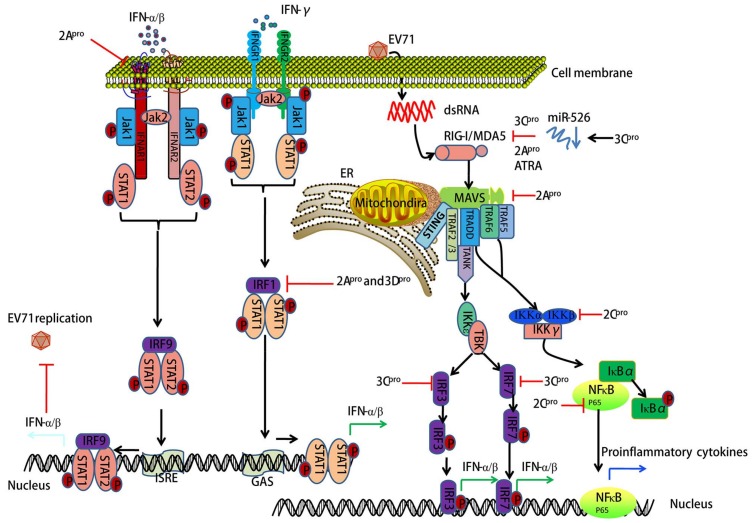
Figure 3.
EV71 inhibits host antiviral innate immunity by targeting IFN, interferon regulatory factor (IRF), and retinoic acid-inducible gene I (RIG-I)-like receptors (RLR)-mediated signaling pathways. Antiviral innate immunity plays a critical role in EV71 infection-induced pathogenesis. EV71-encoded 2Apro, 3Dpro, 3Cpro, and 2Cpro inhibit IFN/STAT (signal transducer and activator of transcription)-mediated type I IFN responses by blocking IFNAR1, IRF-dependent signaling, and by targeting RIG-I, MDA5, MAVS (mitochondrial antiviral-signaling adaptor protein), IKKβ, and NF-κB (p65). On mitochondrial membranes, MAVS is regulated by RLRs such as RIG-I and MDA5, which then interact with the stimulator of interferon genes (STING), the TNF receptor associated factor (TRAF) 2/3, the TNF receptor type 1-associated DEATH domain protein (TRADD), TRAF6, and TRAF5. This process further regulates downstream IκB kinase (IKK)ε/TBK1 and canonical NF-κB signaling. Canonical NF-κB signaling occurs as the IKK complex, consisting of IKKα, IKKβ and IκBα, resulting in the proteasomal degradation of NF-κB inhibitor-α (IκBα) and thus liberating NF-κB to translocate into the nucleus and initiate pro-inflammatory cytokine gene expression. 3Cpro suppresses RIG-I-dependent innate immune responses through down-regulating miR-526. All-trans retinoic acid (ATRA) is a retinoic acid receptor-a (RAR-a) antagonist, which promotes RIG-I signaling against EV71 infection. ER, endoplasmic reticulum.