Abstract
Background
Nausea, vomiting and constipation (OIC) are common adverse effects of acute or chronic opioid use. Naloxegol (25mg) is an approved peripherally active mu-opiate opioid receptor antagonist (PAMORA).
Aim
To compare the effects on pan-gut transit of treatment with codeine, naloxegol, or combination in healthy volunteers.
Methods
We conducted a randomized, double-blind, placebo-controlled, single-center, parallel-group study in 72 healthy opioid-naïve adults, randomized to: codeine (30mg qid), or naloxegol (25mg daily), or codeine and naloxegol (CN), or matching placebo. During 3 days of treatment, we measured gastric emptying (GE) T1/2, colonic filling at 6 hours (CF6), colonic geometric center (GC) at 24 and 48 hours, and ascending colon emptying (ACE) T1/2.
Key Results
Participants were 59.7% females, median BMI 25.0kg/m2 and median age 33.8 years. Codeine significantly retarded GE T1/2, CF6, overall colonic transit and ACE T1/2. There was significant difference (p=0.026) in GE T1/2 between codeine [144.0min (IQR 110.5–238.6)] and naloxegol [95.5min (89.1–135.4)]. There was a significant overall group difference in CF6 (p=0.023), with significant difference (p=0.019) between codeine [11.0% (0.0–45.0)] and naloxegol [51% (18.8–76.2)]. However, no significant differences were found between codeine-treated participants concomitantly receiving placebo or naloxegol.
Conclusions & Inferences
Short-term administration of naloxegol (25mg) in healthy, opioid-naïve volunteers does not reverse the retardation of gastric, small bowel or colonic transit induced by acute administration of codeine. Further studies with naloxegol at higher dose are warranted to assess the ability to reverse the retardation of transit caused by acute administration of codeine in opioid-naïve subjects.
Keywords: opioid, PAMORA, codeine, gastroparesis, constipation
Graphical Abstract
Abbreviated abstract: Nausea, vomiting and constipation (OIC) are common adverse effects of acute or chronic opioid use. We compared the effects on pan-gut transit of treatment with codeine, naloxegol, or combination in healthy volunteers. Short-term administration of naloxegol (25mg) in healthy, opioid-naïve volunteers does not reverse the retardation of gastric, small bowel or colonic transit induced by acute administration of codeine.
Introduction
Opioids are the most commonly used prescription analgesics, with a trend towards increased use over time, for both acute and chronic pain.1,2 Symptoms associated with gastrointestinal dysmotility including nausea, vomiting and constipation are common adverse effects of acute and chronic opioid use.3–5 The effects of opioid agonists on gastrointestinal motility have been studied extensively; morphine was shown to cause a migrating phasic activity mimicking phase III of inter-digestive migrating motor complex in the human small intestine.6 By reducing colonic tone, morphine leads to an increase in storage capability, and slower colonic transit,7 and a decreased number of bowel movements.8 Through their opioid agonist effect on μ and δ receptors, synthetic beta-endorphins lead to a dose-related increase in pyloric phasic pressure activity and induction of intestinal bursts of rhythmic activity, which interrupt normal postprandial motility in healthy individuals.9 Lembo et al. showed that the μ-opioid agonist, fentanyl, inhibits the perception of phasic rectal distention in a dose-dependent fashion.10 In addition, activation of μ and δ opioid receptors reduces active Cl− secretion and passive water movement into the colonic lumen by inhibiting submucosal secretomotor neurons, which can result in stool of more solid consistency.11
Peripherally active μ-opioid receptor antagonists (PAMORA) have emerged as a treatment option for chronic opioid-induced constipation (OIC).12 Naloxegol is a novel PAMORA which is efficacious in the treatment of chronic OIC when administered orally at a dose of 25mg daily.13 It is a polyethylene glycol derivative of naloxol, with significantly less central nervous system (CNS) penetration compared to naloxol.14
There are clinical situations when reversal of the motility effects of μ-opioids could benefit patients who are receiving such medications for a short term, typically for an acute indication. Such patients would be regarded as opioid-naïve. However, the optimal doses of PAMORAs for such indications and the effects of a PAMORA, naloxegol, at the highest dose, 25mg daily approved for use in patients,15 on gastrointestinal and colonic transit retarded by concomitant codeine treatment in opioid-naïve individuals are unclear.
Our study hypothesis is that the approved 25mg daily dose of naloxegol reverses the pan-gut transit retardation induced by codeine. Our aim was to compare the effects on gastrointestinal and colonic transit of treatment with codeine, naloxegol, or combination of both, compared to placebo, in healthy volunteers.
Methods
Participants
We conducted a randomized, double-blind, placebo-controlled, single-center, parallel-group study in 72 healthy volunteers from southeastern Minnesota. Inclusion criteria were: age 18 to 65 years, body mass index (BMI) ≥19 and ≤35 kg/m2, absolute weight between 45 and 100 kg, and absence of any gastrointestinal symptom. Exclusion criteria included: any active medical condition, use of any drug or agent that can alter gastrointestinal transit within 2 weeks preceding and 4 weeks following enrollment, a score of 11 or more on the Hospital Anxiety Depression scale),16 3 or more “yes” responses on the Bowel Disease Questionnaire,17 allergy or hypersensitivity to naloxegol or opioid antagonists, pregnancy or breastfeeding, use of any prescription or nonprescription medication within 7 days prior to enrollment (with the exception of stable doses of thyroid replacement, estrogen replacement, birth control pills or depot injections, and use of acetaminophen on an as needed basis, which were permissible). A negative urine drug screen was documented for all participants, and a negative urine pregnancy test was documented for all female participants of childbearing age.
The study was conducted in the Clinical Research Unit at Mayo Clinic in Rochester, MN. The study was approved by Mayo Clinic Institutional Review Board (IRB# 15-007863 on January 8, 2016), and all participants provided written informed consent. Volunteers were recruited and participated in the studies between July 1, 2016 and May 10, 2017. The study was registered in ClinicalTrials.gov, NCT#02737059.
Randomization
Individuals were randomized (based on computer-generated random sequence without restrictions or stratifications provided by the office of the study statistician) to the following four treatment groups, with 18 participants per group: 1) codeine and placebo (CP); 2) naloxegol and placebo (NP); 3) codeine and naloxegol (CN); and 4) placebo and placebo (PP). Placebo pills were visually matched to the active medications. The doses used were 30mg four times daily for codeine (morphine equivalent: 18mg daily), and 25mg daily for naloxegol. The morning dose of codeine was given simultaneously with the naloxegol first thing in the morning on an empty stomach. All participants ingested one capsule (codeine or matching placebo) four times per day and one capsule of naloxegol or placebo in the morning to ensure blinding.
The randomization code was administered by Mayo Clinic Research Pharmacy; all participants and study staff were blinded until all data were locked and analyzed by the study statistician. Allocation was concealed throughout the study, as the randomization code was only available to the research pharmacist who was located in another section of Mayo Clinic, not in the Clinical Research Unit. All participants, study staff and nurses were blinded to study intervention, as the intervention was coded with a study number.
Experimental Design
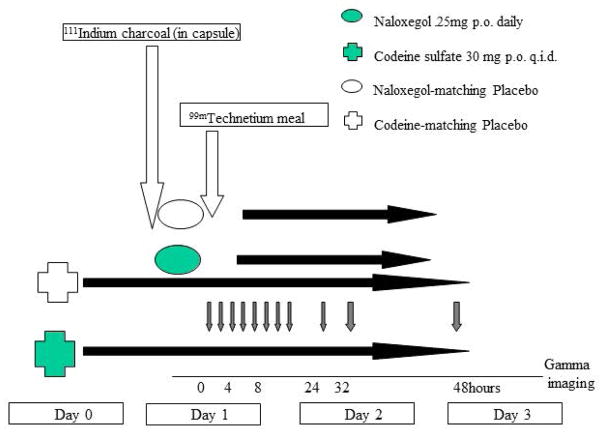
The experimental design is shown in Figure 1.
Figure 1.
Experimental design
Participants were enrolled by the study coordinators and received the study medications from the research pharmacy (not from the study staff) for 3 days starting on day 0 of the study, and underwent detailed scintigraphic testing over 48 hours (starting on day 1).
Measurement of Pan-gastrointestinal Transit
A capsule containing n 111InCl3-labeled activated charcoal particles was used for colonic transit assessment and a 99mTc-labelled egg meal (296kcal, 32% fat) was used to assess gastric emptying and small bowel transit. After ingestion of the radiolabeled meal on day 1, gamma camera images were taken at 0, 15, 30, 60, 90, 120, 150, 180, 210, 240, 300, 360, 420, 480 minutes. Subsequently, images were taken at 24, 28 and 32 hours on day 2, and at 48 hours on day 3. The geometric centers at 4, 8, 24, 28, 32 and 48 hours were estimated using geometric mean of counts in ascending, transverse, descending, and rectosigmoid colon and stool.18,19
The following transit parameters were computed: gastric emptying (GE) T1/2, colonic filling at 6 hours (CF6, a surrogate for combined gastric emptying and small bowel transit), colonic geometric center (GC) at 24 and 48 hours, and ascending colon emptying (ACE) T1/2. All participants underwent an initial medical evaluation during the screening period (within 4 weeks prior to day 0) and a subsequent medical evaluation including assessment for medication compliance and adverse events upon completion of the study on day 3.
Outcome Measures
The primary outcome measures were gastric emptying T1/2, colonic filling (%) at 6 hours, and overall colonic transit summarized as GC at 24 hours. The secondary outcome measures were colonic transit summarized by GC at 4 and 48 hours, ACE T1/2, and percent of standardized meal remaining in the stomach at 2 and 4 hours.
Statistical Analysis
This analysis was conducted with 18 participants per group, except for the codeine-placebo group which consisted of 17 subjects. The analysis was by originally assigned groups and was based on intention-to-treat principles, based on all of the data acquired during the study and with no data imputation. The primary and secondary endpoints were analyzed using a one-way analysis of variance on ranks in view of skewed data distribution. Comparisons between two groups were conducted using Mann-Whitney U test.
Statistical Power with Sample Size in Each Group
The sample size (N=18 per treatment group) provided 80% power to detect the effect sizes listed below for the specific treatment group contrast, assuming the (pooled) variance is known. An analysis of variance provides an estimate of this (pooled) variance based on 68 degrees of freedom. The coefficients of variation (CV%) and corresponding standard deviation (SD) are from recent studies in healthy volunteers using the same measurement methodology18–22 (see Table 1).
Table 1.
Estimated effect size detectable based on n=18 subjects per group
| Response | Mean (implied using SD and CV) | SD | CV (%) | Detectable § Difference (%) | Detectable § Difference (Δ) |
|---|---|---|---|---|---|
| GE T1/2 (min) | 120 | 30 | 25 | 24 | 29 min |
| GE @ 2 hr (%) | 57 | 17 | 30 | 28 | 16 % |
| CF6 @ 6hr ( %) | 64 | 27 | 42 | 41 | 26 % |
| GC @ 24hr | 2.48 | 0.99 | 40 | 39 | 0.97 GC units |
Based on 80% power and N=18 per group
The magnitude of change detectable is considered clinically relevant. For example, the ~30-minute difference in gastric emptying that could be detected between treatment groups would be clinically relevant, since it would be associated with a difference in 100 kcal ingested at a buffet meal, or 150 kcal ingested to achieve fullness in a laboratory-based nutrient drink test.23 Similarly, prior studies showed that a decrease in colonic transit geometric center of 0.97 units at 24 h is associated with a change in stool consistency of ~0.6 units on the Bristol stool form scale and ~0.5 stools per day,24 while an increase of the colon GC24 of 1.8 GC units is associated with significant diarrhea, as in carcinoid syndrome.25 Table 1 shows the minimum effect sizes that could be detected with the stated power; larger differences observed would even provide higher clinical effects.
Results
Participants
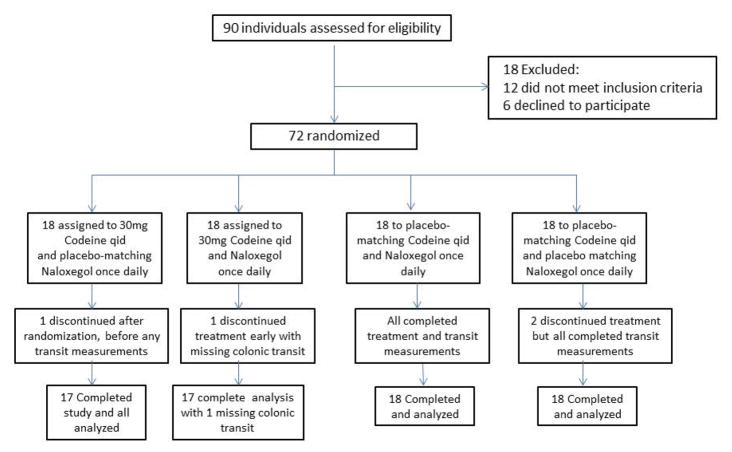
Figure 2 shows the CONSORT flow chart describing participant disposition. Among the participants, 59.7% were females (n=43), with median BMI of 25.0 kg/m2 and median age of 33.8 years. Detailed demographics are provided in Table 2A, which shows there were no significant differences in the four groups. All the participants who were randomized received intended treatment (except one patient who dropped out during day 1 of dosing), and all their data were analyzed for primary and secondary outcomes. One participant (randomized to codeine and placebo) dropped out of the study during the first day of dosing and no measurements were obtained.
Figure 2.
CONSORT flow chart of participant disposition
Table 2A.
Patient demographics for the 4 treatment groups
| Data, median (IQR) | Codeine-Naloxegol | Codeine-Placebo | Naloxegol-Placebo | Placebo-Placebo |
|---|---|---|---|---|
| N | 18 | 17* | 18 | 18 |
| Age (years) | 30.6 (23.1–53.9) | 28.8 (25.6–49.7) | 28.1 (23.8–44.4) | 30.7 (25.1–49.3) |
| BMI (kg/m2) | 25.6 (21.7–29.3) | 25.0 (22.3–28.7) | 24.4 (22.2–29.1) | 23.4 (22.0–28.0) |
One participated withdrew before any measurements
Effects on Gastric and Small Bowel Transit
All transit data are provided in Table 2B.
Table 2B.
Gastric emptying, colonic filling and colonic transit measurements in the four treatment groups
| Median(IQR) | Codeine-Naloxegol | Codeine-Placebo | Naloxegol-Placebo | Placebo-Placebo |
|---|---|---|---|---|
| GE T1/2, min | 144.7 (103.4–218.6) | 144.0 (110.5–238.6) | 95.5 (89.1–135.4) | 115.6 (99.5–150.5) |
| CF at 6h, % | 50.0 (0.0–48.5) | 11.0 (0.0–45.0) | 51.0 (18.8–76.2) | 37.5 (19.0–64.8) |
| GC8 | 1.0 (0.5–1.1) | 0.9 (0.4–1.0) | 1.0 (0.1–1.2) | 1.3 (1.0–1.5) |
| GC24 | 1.6 (1.3–2.4) | 1.7 (1.3–2.4) | 2.3 (1.6–2.6) | 2.6 (2.0–3.5) |
| GC48 | 2.9 (2.2–4.5) | 3.1 (2.4–4.5) | 4.1 (3.3–4.7) | 4.6 (3.6–5.0) |
| ACE T1/2, h | 20.3 (16.5–30.8) | 20.5 (16.6–27.8) | 17.0 (15.6–18.8) | 13.5 (8.5–19.5) |
IQR=interquartile range; GE=gastric emptying; CF=colonic filling; GC=geometric center; ACE=ascending colon emptying
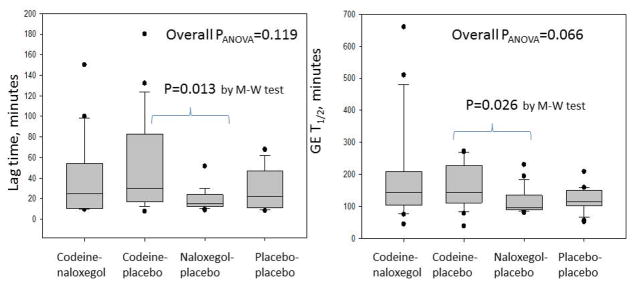
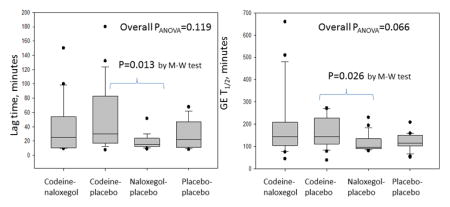
There was non-significant overall difference in GE T1/2 between the 4 groups (p=0.066). As expected, codeine numerically retarded gastric emptying [CP: 144.0 (110.5–238.6) min] compared to the placebo group [PP: 115.6 (99.5–150.5)] though this did not reach significance (p=0.083). In addition, there were significant differences (p=0.026) in GE T1/2 between CP (144.0min, 110.5–238.6) and NP (95.5min, 89.1–135.4), but not between NP and PP groups (Figure 3).
Figure 3.
Analysis of variance of the lag time and gastric emptying (GE) T1/2 among the 4 groups: codeine-naloxegol, codeine-placebo, naloxegol-placebo, placebo-placebo.
M-W=Mann-Whitney U test
There were no significant differences between the 4 groups (p=0.119) in GE lag time, but direct comparison between CP (30.0, 15.8–83.3) and NP (15.0, 12.5–25.5) showed a significant difference (p=0.013) (Figure 3).
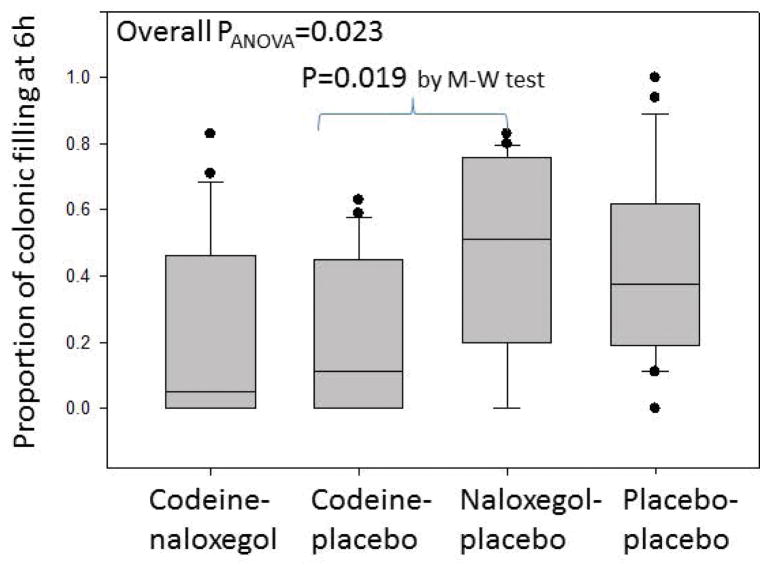
There was a significant overall group difference in percent colonic filling at 6 hours (CF6) (p=0.023), with significant difference (p=0.019) between CP (11.0% filling at 6 hours, 0.0–45.0%) and NP (51%, 18.8–76.2%) (Figure 4).
Figure 4.
Analysis of variance of the proportion of colonic filling at 6 hours among the 4 groups: codeine-naloxegol, codeine-placebo, naloxegol-placebo, placebo-placebo.
M-W=Mann-Whitney U test
Note that, with reference to the primary aim of the study, there were no significant differences in GE T1/2 and CF6 between CN and CP groups, indicating that naloxegol did not reverse the effects of codeine at the dosages administered.
Colonic Transit and Evaluation of Effect of Naloxegol on Retardation of Gut Transit by Codeine
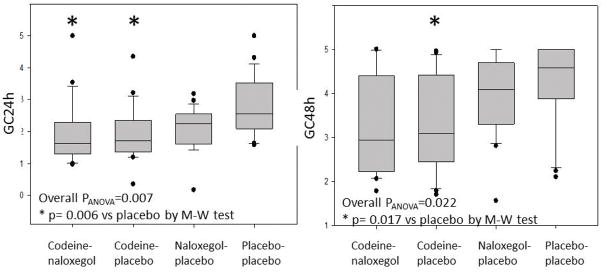
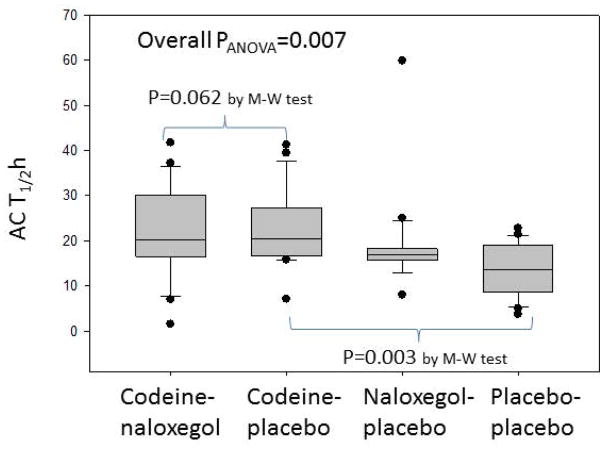
There were no significant differences in geometric center at 24 or 48 hours between CP and CN groups (Figure 5). As expected, codeine significantly retarded colonic transit and ACE T1/2 (Figure 6). Naloxegol did not accelerate colonic transit relative to codeine treated groups.
Figure 5.
Analysis of variance of geometric center (GC)24h and GC48h among the 4 groups: codeine-naloxegol, codeine-placebo, naloxegol-placebo, placebo-placebo.
M-W=Mann-Whitney U test
Figure 6.
Analysis of variance of ascending colonic transit (ACT) T1/2h among the 4 groups: codeine-naloxegol, codeine-placebo, naloxegol-placebo, placebo-placebo.
M-W=Mann-Whitney U test
Adverse Effects
No serious adverse events (AE) were reported and there was no significant difference in the rate of occurrence of individual AEs between the 4 groups (Table 3). The most commonly reported AEs were: constipation (n=21), nausea (n=14), headache (n=14), fatigue (n=11) and dizziness (n=9). The 4-group comparisons for constipation and nausea were not significant (p=0.094 and 0.069, respectively) though they appeared to be numerically higher with codeine treatment.
Table 3.
Adverse effects observed in each treatment group
| Number (%) | Codeine-Naloxegol | Codeine-Placebo | Naloxegol-Placebo | Placebo-Placebo |
|---|---|---|---|---|
| N | 18 | 17 | 18 | 18 |
| Constipation | 8 (44%) | 7 (41%) | 2 (11%) | 4 (22%) |
| Nausea | 7 (39%) | 4 (23%) | 1 (6%) | 2 (11%) |
Discussion
In opioid-naïve healthy volunteers without OIC or constipation, the short-term administration of codeine retards transit pan-gut transit compared to placebo, and numerically but not significantly (p=0.083) slows gastric emptying compared to placebo. However, naloxegol, 25mg daily, does not reverse the retardation of small bowel or colonic transit induced by the acute administration of codeine. These results could be explained by the relatively low dose of naloxegol, given the modest potency of naloxegol as a μ-opioid receptor antagonist compared to other opioid receptor antagonists such as naloxone.14 It was previously shown that the prolongation of orocecal transit time resulting from acute administration of 5mg of IV morphine (prorated based on 70kg weight) was significantly reduced by naloxegol, only when administered at doses of 125mg or higher;26 thus, a dose of 25mg may be too small to reverse the GI transit retardation caused by opioid agonists in a short-term course. Higher doses (the dose range tested in one study with acute administration was 8, 15, 30, 60, 125, 250, 500, and 1000 mg) were shown to be generally safe, with low risk of reversal of CNS-mediated analgesia; in addition, greater reversal of morphine-induced delay in OCTT occurred with increasing naloxegol dose.27 The half-maximal effect (iAUC50) of naloxegol effects on oro-cecal transit times (OCTT) was observed at plasma concentrations associated with doses of 15–30 mg naloxegol.27 However, this effect was observed with transit of lactulose, a liquid substrate rather than solids, and it is clear that opioid-induced disturbances of solid transit through the stomach and colon, in particular, are the most relevant. The OCTT with lactulose does not test either of these functions.
Higher doses of naloxegol, therefore, may be needed to prevent or treat constipation resulting from acute administration of narcotic analgesics. Further studies with naloxegol at higher dose are warranted to assess its ability to reverse the retardation of GI transit caused by acute administration of codeine. An example of clinical applicability of this approach, if proven effective in acute pain, would be to start an appropriate dose of naloxegol prior to narcotics following a surgical procedure known to cause significant pain, such as orthopedic surgeries, when opioid analgesics are contemplated. However, higher doses are not currently approved for use in human subjects.
We ensured that the duration of administration of naloxegol was sufficient to achieve pharmacological activity, since naloxegol is rapidly absorbed with mean time to maximum plasma concentration of <2 hours, and following once-daily administration, steady state is achieved within 2–3 days.13
Despite the limited efficacy of the dose used in this study when administered short term, naloxegol offers the advantage of an oral administration with minimal CNS penetration, thus preserving centrally mediated analgesia delivered by opioid receptor agonists.14 Our study and prior studies28 have shown that naloxegol is well tolerated and safe for acute and chronic use. However, opioid-naïve users needed 125mg of naloxegol to reverse the effects of opioids on transit;26 this is in contrast to the 12.5 to 25mg naloxegol dose needed to treat OIC in chronic opioid users.
An analysis of efficacious doses of PAMORAs for the inhibition of opioid analgesic effects on small bowel and colonic transit, as well as on symptoms related to OIC or postoperative ileus, shows that the doses required for efficacy are typically 1 log lower for chronic opioid-treated patients compared to opioid-naïve patients.29 Our results on naloxegol are consistent with similar findings in prior studies on other PAMORAs: the approved dose of methylnaltrexone (0.30mg/kg SQ) to treat OIC in patients on chronic opioid therapy does not appear to reverse the inhibitory effect of codeine on colonic motility in opioid-naïve healthy volunteers, and a higher dose may be needed in that setting.30 Indeed, a pharmacodynamics trial demonstrated reversal of morphine-induced delay in orocecal transit time (measured by lactulose-breath hydrogen) with a methylnaltrexone dose 50% higher (0.45mg/kg) than the approved dose of 0.30mg/kg.31 It is however, worth noting that the effect of 0.45mg/kg methylnaltrexone on acute, morphine-delayed colonic transit was not investigated and may conceivably be higher than the dose that revered effect on orocecal transit of liquid.
Similarly, alvimopan was shown to be effective at treating OIC in patients with chronic non-cancer pain on narcotics at doses as low as of 0.5mg orally BID,32 whereas, a higher dose (12mg orally BID) was used to reverse codeine’s inhibitory effect on colonic and small bowel transit in healthy opioid-naïve volunteers receiving a short course of codeine.33 Repeated opioid administration may lead to opioid receptor downregulation or decoupling from their downstream intracellular signaling activity, leading to the need for higher dosage of agonist to achieve the same analgesic effect and, possibly, a lower dosage of μ-opioid antagonist to reverse the effect of chronic μ-opioid treatment. An alternative hypothesis is that the effects of opioid agonists are more pronounced in opioid-naïve individuals, leading to more solid consistency of intraluminal content and slower transit. These effects may be more difficult to reverse by opioid antagonists in an acute setting.
These observations, including our results, carry important clinical implications, as they further clarify the physiologic mechanism of action of PAMORAs in different clinical scenarios. The data suggest that a higher dose may indeed be needed for opioid-naïve patients who develop bowel dysfunction secondary to a new exposure to opioid analgesics. Thus PAMORAs, including naloxegol, may be beneficial to prevent and treat constipation in these patients, if the appropriate higher dose is used. Furthermore, the risk of opioid withdrawal, which is small when PAMORAs are used, is likely to be even smaller in opioid-naïve individuals receiving a short course of narcotics, and higher doses of PAMORAs may be safe.
There are many strengths in this study including the randomized, double-blind, placebo-controlled, single-center, parallel-group study design, the use of a clinically relevant dose of codeine (30mg qid), and the use of the gold standard (scintigraphy) to assess GI and colonic transit. These attributes suggest the data are generalizable to healthy, opioid-naïve people. In addition, the dose of codeine administered was associated with a ~30 minute difference in GE T1/2 and a difference in GC24 of 0.9 GC unit, compared to placebo. Therefore the study was appropriately powered to be able to detect pharmacodynamics effect of codeine and potentially, the reversal or inhibition of these effects by naloxegol.
Limitations include the use of a relatively low opioid agonist dose, compared to the typical doses used in clinical practice to treat acute pain in opioid-naïve patients. In addition, the study lacks data on the effect of naloxegol use beyond 3 days and the effects of different doses of naloxegol. Our current study was also limited by the dose approved for use in human subjects, but it provided the important information that, at this highest approved dose, naloxegol was not effective in reversing the inhibition of pan-gut transit resulting from the use of an opioid at standard, clinically applicable doses. Therefore, future studies are needed to assess the effects of higher naloxegol doses and different treatment durations on the GI transit altering effect of opioid agonists in patients who are opioid-naïve, as well as in subjects on high dose opioids chronically for a single condition.
Conclusion
In opioid-naïve healthy volunteers without OIC or constipation, the short-term administration of naloxegol (25mg/day) accelerates gastric and small bowel transit compared to codeine, which retards transit compared to placebo. However, naloxegol, 25mg/day, does not reverse the retardation of gastric, small bowel or colonic transit induced by acute administration of codeine, 30mg qid. Further studies with naloxegol at higher dose are warranted to assess the ability to reverse the retardation of transit caused by acute administration of codeine.
Key Points.
State of current knowledge
Opioids retard gastrointestinal and colonic transit and induce symptoms such as nausea, vomiting and constipation
Peripherally-acting mu-opioid receptor antagonists (PAMORA) are approved for the treatment of chronic opioid-induced constipation
Effects of a PAMORA, naloxegol, at the highest dose approved, on gastrointestinal and colonic transit of solids in opioid-naïve individuals are unclear.
Results of current paper
Over a short term treatment, naloxegol 25mg daily does not reverse effects of codeine at standard dosing (30mg qid) on gastrointestinal or colonic transit of solids
Importance of results in neurogastroenterology
Currently approved doses of PAMORAs are based on treatment of patients with chronic OIC, and may not be efficacious for the treatment of acute gastrointestinal motility disturbances resulting from mu-opioid therapy
Further studies with naloxegol at higher dose are warranted to assess the ability to reverse the retardation of pan-gut transit caused by acute administration of codeine in opioid-naïve subjects.
Acknowledgments
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding: This study was supported by a grant from AstraZeneca. Dr. Camilleri was supported during the conduct of the studies by R01-DK92179 and R56-DK67071 grants from National Institutes of Health. This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Clinical trial registration: ClinicalTrials.gov NCT#02737059
Disclosures: Dr. Camilleri has served as a consultant (with the consulting fee going to his employer, Mayo Clinic) for AstraZeneca.
The other authors have no personal interests to declare.
Guarantor of the article:
Dr. Michael Camilleri takes responsibility for the integrity of this work as a whole, from inception to published article.
Authors’ contributions:
Houssam Halawi – research fellow, patient care, authorship of manuscript
Priya Vijayvargiya - research fellow, patient care, authorship of manuscript
Irene Busciglio – study coordinator, patient recruitment, database management
Ibironke Oduyebo - research fellow, patient care, authorship of manuscript
Disha Khemani - research fellow, patient care, authorship of manuscript
Michael Ryks – technical support for the studies
Deborah Rhoten – technical support for the studies
Duane Burton – database management
Lawrence A. Szarka – staff physician, patient studies
Andres Acosta – staff physician, patient studies
Michael Camilleri - Staff supervisor, review of patient data, test results and authorship
All authors approved the final manuscript.
References
- 1.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(2 Suppl):S63–S88. [PubMed] [Google Scholar]
- 2.Nicholson BD. Economic and clinical burden of opioid-induced nausea and vomiting. Postgrad Med. 2017;129:111–117. doi: 10.1080/00325481.2017.1243004. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson M, Poulsen JL, Brock C, et al. Opioid-induced bowel dysfunction in healthy volunteers assessed with questionnaires and MRI. Eur J Gastroenterol Hepatol. 2016;28:514–524. doi: 10.1097/MEG.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 4.Poulsen JL, Nilsson M, Brock C, Sandberg TH, Krogh K, Drewes AM. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil. 2016;22:282–291. doi: 10.5056/jnm15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ketwaroo GA, Cheng V, Lembo A. Opioid-induced bowel dysfunction. Curr Gastroenterol Rep. 2013;15:344. doi: 10.1007/s11894-013-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borody TJ, Quigley EM, Phillips SF, et al. Effects of morphine and atropine on motility and transit in the human ileum. Gastroenterology. 1985;89:562–570. doi: 10.1016/0016-5085(85)90452-4. [DOI] [PubMed] [Google Scholar]
- 7.Steadman CJ, Phillips SF, Camilleri M, Talley NJ, Haddad A, Hanson R. Control of muscle tone in the human colon. Gut. 1992;33:541–546. doi: 10.1136/gut.33.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman PN, Krevsky B, Malmud LS, et al. Role of opiate receptors in the regulation of colonic transit. Gastroenterology. 1988;94:1351–1356. doi: 10.1016/0016-5085(88)90673-7. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M, Malagelada JR, Stanghellini V, Zinsmeister AR, Kao PC, Li CH. Dose-related effects of synthetic human beta-endorphin and naloxone on fed gastrointestinal motility. Am J Physiol. 1986;251:G147–G154. doi: 10.1152/ajpgi.1986.251.1.G147. [DOI] [PubMed] [Google Scholar]
- 10.Lembo T, Naliboff BD, Matin K, et al. Irritable bowel syndrome patients show altered sensitivity to exogenous opioids. Pain. 2000;87:137–147. doi: 10.1016/S0304-3959(00)00282-7. [DOI] [PubMed] [Google Scholar]
- 11.Galligan JJ, Sternini C. Insights into the role of opioid receptors in the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:363–378. doi: 10.1007/164_2016_116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pergolizzi JV, Jr, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107–119. doi: 10.2147/PPA.S78042. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bui K, Zhou D, Xu H, Floettmann E, Al-Huniti N. Clinical pharmacokinetics and pharmacodynamics of naloxegol, a peripherally acting μ-opioid receptor antagonist. Clin Pharmacokinet. 2017;56:573–582. doi: 10.1007/s40262-016-0479-z. [DOI] [PubMed] [Google Scholar]
- 14.Floettmann E, Bui K, Sostek M, Payza K, Eldon M. Pharmacologic profile of naloxegol, a peripherally acting μ-opioid receptor antagonist, for the treatment of opioid-induced constipation. J Pharmacol Exp Ther. 2017;361:280–291. doi: 10.1124/jpet.116.239061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center for Drug Evaluation and Research. [Accessed October 20, 2017];NDA 204760 Movantik (naloxegol) tablets, AstraZeneca Pharmaceuticals LP. 2014 Sep 16; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/204760Orig1s000OtherR.pdf.
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ, Phillips SF, Melton LJ, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri M, Colemont LJ, Phillips SF, et al. Human gastric emptying and colonic filling of solids characterized by a new method. Am J Physiol. 1989;257:G284–G290. doi: 10.1152/ajpgi.1989.257.2.G284. [DOI] [PubMed] [Google Scholar]
- 19.Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med. 1997;38:1807–1810. [PubMed] [Google Scholar]
- 20.Camilleri M, Zinsmeister AR. Towards a relatively inexpensive, noninvasive, accurate test for colonic motility disorders. Gastroenterology. 1992;103:36–42. doi: 10.1016/0016-5085(92)91092-i. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Zinsmeister AR, Greydanus MP, Brown ML, Proano M. Towards a less costly but accurate test of gastric emptying and small bowel transit. Dig Dis Sci. 1991;36:609–615. doi: 10.1007/BF01297027. [DOI] [PubMed] [Google Scholar]
- 22.von de Ohe MR, Camilleri M. Measurement of small bowel and colonic transit: indications and methods. Mayo Clinic Proc. 1992;67:1169–1179. doi: 10.1016/s0025-6196(12)61147-1. [DOI] [PubMed] [Google Scholar]
- 23.Halawi H, Camilleri M, Acosta A, Vazquez-Roque MI, Oduyebo I, Burton DD, Busciglio IA, Zinsmeister AR. Relationship of gastric emptying or accommodation with satiation, satiety and postprandial symptoms in health. Am J Physiol Gastrointest Liver Physiol. 2017 Aug 3; doi: 10.1152/ajpgi.00190.2017. ajpgi.00190.2017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinsmeister AR, Burton D, Camilleri M. Pharmacodynamic and clinical endpoints for functional colonic disorders: statistical considerations. Dig Dis Sci. 2013;58:509–518. doi: 10.1007/s10620-012-2369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von der Ohe M, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329:1073–1078. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 26.Neumann T, Van Paaschen H, Marcantonio A, Song D, Morrison P, Eldon M. Evaluation of PEG-naloxol (NKTR-118) as an oral peripheral opioid antagonist in healthy male subjects: a double-blind, placebo-controlled, dose escalation crossover study. Presented at the 36th Annual American College of Clinical Pharmacology Meeting; San Francisco, CA. September 9–11, 2007. [Google Scholar]
- 27.Eldon MA, Kugler AR, Medve RA, Bui K, Butler K, Sostek M. Safety, tolerability, pharmacokinetics, and pharmacodynamic effects of naloxegol at peripheral and central nervous system receptors in healthy male subjects: A single ascending-dose study. Clin Pharmacol Drug Dev. 2015;4:434–441. doi: 10.1002/cpdd.206. [DOI] [PubMed] [Google Scholar]
- 28.Webster L, Tummala R, Diva U, Lappalainen J. A 12-week extension study to assess the safety and tolerability of naloxegol in patients with noncancer pain and opioid-induced constipation. J Opioid Manag. 2016;12:405–419. doi: 10.5055/jom.2016.0360. [DOI] [PubMed] [Google Scholar]
- 29.van Malderen K, Halawi H, Camilleri M. Insights on efficacious cases of PAMORAs for patients on chronic opioid therapy or opioid-naïve patients. Neurogastroenterol Motil. 2017 doi: 10.1111/nmo.13250. in press. [DOI] [PubMed] [Google Scholar]
- 30.Wong BS, Rao AS, Camilleri M, et al. The effects of methylnaltrexone alone and in combination with acutely administered codeine on gastrointestinal and colonic transit in health. Aliment Pharmacol Ther. 2010;32:884–893. doi: 10.1111/j.1365-2036.2010.04422.x. [DOI] [PubMed] [Google Scholar]
- 31.Yuan CS, Foss JF, O’Connor M, Toledano A, Roizen MF, Moss J. Methylnaltrexone prevents morphine-induced delay in orocecal transit time without affecting analgesia: a double-blind randomized placebo-controlled trial. Clin Pharmacol Ther. 1996;59:469–475. doi: 10.1016/S0009-9236(96)90117-4. [DOI] [PubMed] [Google Scholar]
- 32.Webster L, Jansen JP, Peppin J, et al. Alvimopan, a peripherally acting mu-opioid receptor (PAM-OR) antagonist for the treatment of opioid-induced bowel dysfunction: results from a randomized, double-blind, placebo-controlled, dose-finding study in subjects taking opioids for chronic non-cancer pain. Pain. 2008;137:428–440. doi: 10.1016/j.pain.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Gonenne J, Camilleri M, Ferber I, et al. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol. 2005;3:784–791. doi: 10.1016/s1542-3565(05)00434-9. [DOI] [PubMed] [Google Scholar]