Figure 2. Synthesis and characterization of S-nitrosothiol poly(propylene sulfide) nanoparticles (SNO-NP).
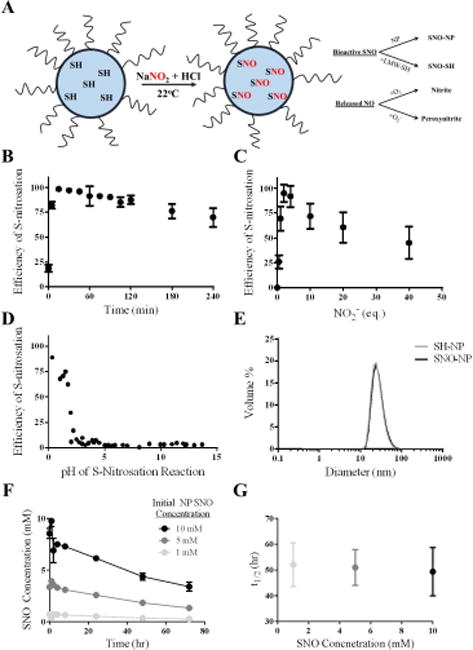
A) Schematic of micellar structure of Pluronic-stabilized, poly(propylene sulfide) NP containing free core thiols that become S-nitrosated using an acidified sodium nitrite protocol at room temperature. SNO on the SNO-NP can either be retained on the NP or can be transnitrosated to a low molecular weight thiol (LMW-SH); NO that is released from the SNO-NP will be either oxidized with oxygen to nitrite, or will interact with superoxide and form peroxynitrite. B) Efficiency of S-nitrosation reaction, defined as the amount of S-nitrosothiol that can be generated per amount of starting free thiol concentration, reaches a maximum around 15 minutes. C) Efficiency of S-nitrosation reaches a maximum at 2–4 eq. sodium nitrite to free core thiols. D) Low pH is required for the S-nitrosation reaction with sodium nitrite and free core thiols. E) SNO-NP are stable and the same size as unmodified NP, indicating that the Pluronic-stabilized, poly(propylene sulfide) NP structure remains stable under these conditions. F) SNO decay from SNO-NP at different starting SNO concentrations over 3 d. G) The approximately 50 hour half-life of the SNO release from SNO-NP is constant at 37°C regardless of starting SNO concentration. n=3 samples per group.
