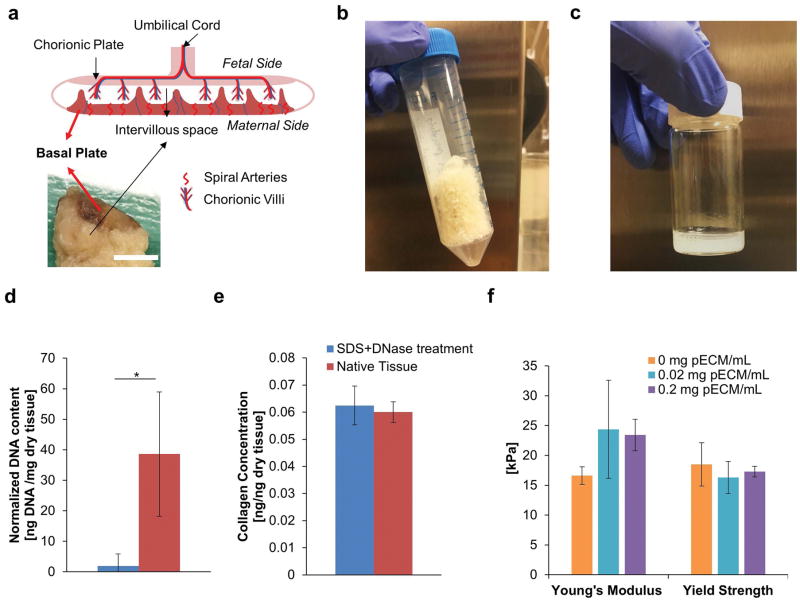
Figure 1. Development of Decellularized Placental ECM (pECM).
(a) Anatomy of Ex Vivo Placenta. Top image illustrates the major structures of human placenta and bottom image demonstrated a cross section of human placenta sample. Red arrows indicated the location of basal plate. Scale bar= 10 mm. (b) Representative Image of Lyophilized pECM. The tissue of basal plate was lyophilized for long term storage after it has been decellularized. The lyophilized pECM was a porous scaffold with white-yellow appearance. (c) Representative Image of Solubilized pECM. The lyophilized pECM was solubilized in pepsin under acidic condition at a desired concentration to be bioprinted later. (d) DNA Content of Solubilized ECM. After treatments of SDS and DNase (blue), 95% of the DNA from the native tissue was removed (red, n=3). (e) Collagen Content of Solubilized ECM. There was no significant difference between the collagen contents of solubilized decellularized and native tissue, which suggested the integrity of the ECM was well-preserved after decellurlarization treatment (n=3). (f) Effect of pECM on the Mechanical Properties of Bioprinted Constructs (n=6). Our results showed that the addition of pECM into bioprinted GelMA constructs did not alter the Young’s modulus and Yield Strength significantly.