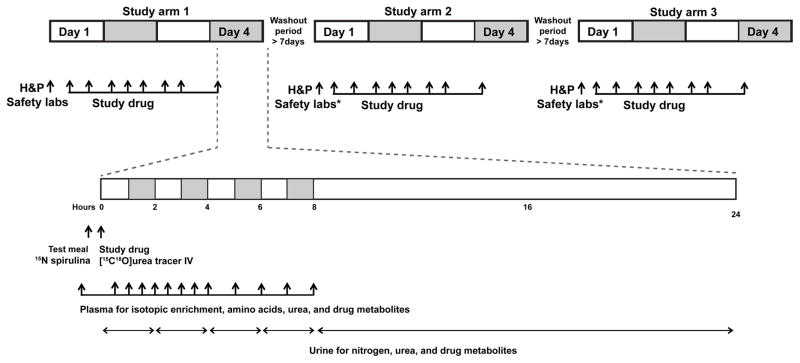
Figure 2. Study design and procedures.
Every subject was crossed-over to receive all three study medications, i.e., phenylbutyrate, benzoate and combination of phenylbutyrate and benzoate at half the dose. (H&P - history and physical examination; Safety labs - complete blood count, comprehensive metabolic panel, plasma ammonia, and urinalysis; * safety labs in study arms 2 and 3 were repeated only if abnormalities were noted on safety labs from the Day 4 of the preceding study arm or if the washout period was greater than 28 days from the preceding treatment arm). On day 4, after consuming the test meal (0.4 g protein/kg) labeled with 15N Spirulina, the subjects were administered the corresponding drugs and a urea tracer (13C18O urea). Blood and urea were sampled as indicated.