Abstract
It has become increasingly clear that pituitary hormones that have traditionally been seen as regulators of single bodily processes, including endocrine functions, have additional roles in physiology. The global deletion of these hormones or their receptors in mice has yielded unexpected skeletal and metabolic phenotypes, which have been confirmed through traditional pharmacologic approaches (Zaidi 2007). Initial reports showed that thyroid-stimulating hormone receptors (TSHRs) were expressed on bone cells and that their haploinsufficiency in Tshr+/− mice caused osteopenia without affecting the thyroid function (Abe, et al. 2003). Likewise, receptors for follicle-stimulating hormone (FSHRs) are expressed in both skeletal cells and adipose tissue, where they regulate body composition (Feng, et al. 2017; Liu, et al. 2017; Liu, et al. 2015; Sun, et al. 2006). However, due to feedback circuitry, it has often been difficult to tease out specific actions of pituitary hormones on these newly described targets from known actions of the hormones they produce (Blair, et al. 2011). With emerging evidence for such additional roles of pituitary hormones in integrative physiology, new avenues for therapy have been unmasked (Liu et al. 2017). In this review, we discuss these newly discovered actions of pituitary hormones and their physiologic and therapeutic relevance.
Keywords: follicle-stimulating hormone, adrenocorticotropic hormone, growth hormone, oxytocin, vasopressin
Evolutionary Considerations
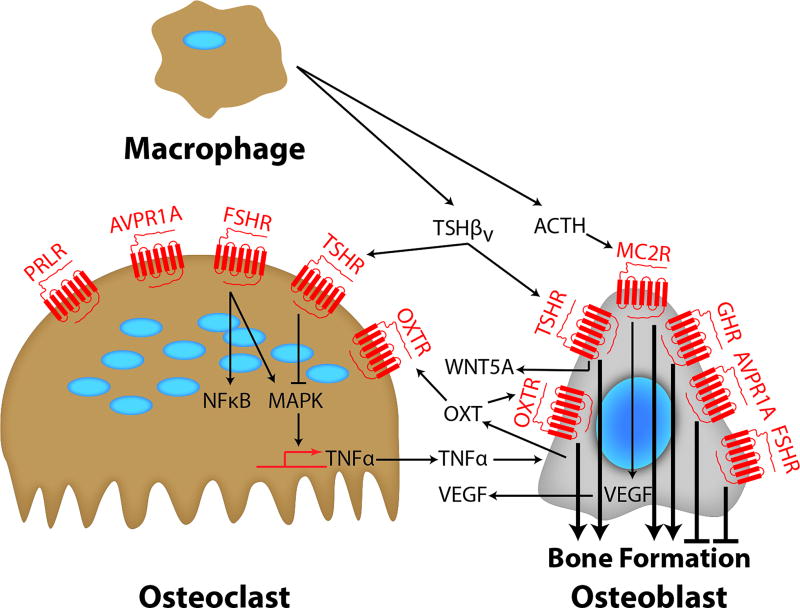
The most obvious example of a pituitary hormone being part of a widely distributed G-protein coupled receptor (GPCR) system is adrenocorticotropic hormone (ACTH), known to participate in cell differentiation in several contexts. This distributed function is nonetheless overshadowed by its pituitary-adrenal signaling function. Tissue-specific, regulated proteolytic processing of a single large prohormone, proopiomelanocortin (POMC), yields ligands that act on five melanocortin receptors (MC1R – MC5R), including the ACTH receptor (MC2R), which regulates cellular functions such as pigment production, appetite, and sexual function. While ACTH is the predominant product in the anterior pituitary, three melanotropins and β-endorphin are synthesized from the same precursor at other sites (Blair et al. 2011). There are reports of ACTH production by human macrophages/monocytes (Pallinger and Csaba 2008), making it possible that MC2Rs in bone may be activated by local, instead of pituitary-derived ACTH (Figure 1). Abundant expression of the MC2R has also been documented in bone forming units in adult mice (Isales, et al. 2010). Similarly to its action in the vertebrate adrenal cortex, ACTH acts on osteoblastic MC2Rs to induce the expression of vascular endothelial growth factor (VEGF) (Zaidi, et al. 2010) (Figure 1). This is the likely basis of ACTH action in preventing glucocorticoid-induced osteonecrosis, as assessed in a rabbit model (Zaidi et al. 2010).
Figure 1. Endocrine Control of Bone Remodeling.
Bone formation is regulated by signals from pituitary hormones. GH, ACTH, FSH, TSH, PRL, OXT and AVP regulate both osteoclasts and osteoblasts directly through GPCRs. Certain ligands, namely OXT, TSHβv and ACTH, are also produced in bone marrow by macrophages and/or osteoblasts.
TSH and FSH, together with chorionic gonadotropin (CG) and luteinizing hormone (LH), are a group of heterodimeric proteins that share a common α-chain, with their differing β-chains determining specificity. These hormones are particularly interesting in that simpler phyla have distributed functions. In the primitive nervous system of coelenterates, which have no endocrine glands, a TSHR family gene is expressed widely and shows the intron–exon structure found in mammals (Vibede, et al. 1998). In bony fish, abundant TSHR is expressed in the thyroid, but the receptor is also detectable in ovaries, heart, muscle, and brain (Kumar, et al. 2000). Fish gonads also express the LH/CG receptor (LHCGR) and FSH receptor (FSHR), with multiple differently processed forms of the FSHR that bind both FSH and LH (Bogerd, et al. 2005; Kobayashi and Andersen 2008). Low-level expression of the FSHR and TSHR has been reported in the spleen and bone marrow, respectively (Kumar, et al. 2001; Vincent, et al. 2009a).
TSH and Bone Mass Regulation
In 2003, Abe and coworkers documented direct actions of TSH on bone that were independent of thyroid hormones (Abe et al. 2003; Novack 2003). Three key findings separated the known action of high thyroid hormone on bone from the newly identified TSH effects. First, TSHR haploinsufficiency in heterozygotic Tshr−/− mice was found to yield an osteoporotic phenotype with increased osteoclast formation ex vivo, while thyroid hormone levels remained unchanged. Second, the osteoporosis noted in Tshr null mice could not be explained by low thyroid hormone levels, as high, rather than low levels have been implicated in bone loss. Third, and importantly, skeletal runting, but not the osteoporotic phenotype, was reversed by thyroid hormone replacement that rendered Tshr−/− mice euthyroid (Abe et al. 2003).
The idea therefore emerged that TSH acted on bone independently of thyroid hormones, and that the osteoporosis of hyperthyroidism may, in part, result from low TSH (Zaidi, et al. 2006). This latter hypothesis was studied in wild type and Tshr−/− mice that were rendered either euthyroid or hyperthyroid by implanting thyroxine (T4) pellets (Baliram, et al. 2012). Hyperthyroid Tshr−/− mice suffered greater bone loss than wild type mice that were equally hyperthyroid (Baliram et al. 2012). This suggested that absent TSH signaling in Tshr−/− mice itself contributes to hyperthyroid bone loss.
Consistent with the high-turnover osteoporosis seen in hyperthyroidism, Tshr−/− mice and hyt/hyt mice, in which the TSH receptor is naturally defective, showed evidence of increased osteoclast formation (Britto, et al. 1994; Sun, et al. 2008). Studies further showed that recombinant TSH attenuated the genesis, function and survival of osteoclasts in vitro (Abe et al. 2003; Ma, et al. 2009). Accordingly, osteoclastogenesis was inhibited when the constitutively activated TSHR was overexpressed in osteoclast precursors or an activating TSHR antibody was injected (Hase, et al. 2006; Ma, et al. 2011; Sun et al. 2008). Human data for a direct action of TSH on bone is equally compelling – a single subcutaneous injection of recombinant human TSH lowered serum C-terminal telopeptide (CTX) in post-menopausal women within 2 days (Mazziotti, et al. 2005). In no instance of TSH replacement did thyroid hormones increase. We also know that these anti-osteoclastogenic actions of TSH are mediated by reduced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and Janus N-terminus kinase (JNK) activation, as well as indirectly, through diminished tumor necrosis factor α (TNFα) production (Abe et al. 2003; Hase et al. 2006). The effect of TSH on TNFα synthesis is mediated transcriptionally by binding of two high mobility group box proteins, HMGB1 and HMGB2, to the Tnfα gene promoter (Yamoah, et al. 2008). Thus, TNFα production is upregulated in osteoporotic Tshr−/− mice (Abe et al. 2003), and the genetic deletion of Tnfα in these mice reverses the osteoporosis and the bone remodeling defects, proving that the Tshr−/− phenotype is mediated by TNFα, at least in part (Sun, et al. 2013).
TSH inhibits osteoblastogenesis in bone marrow-derived cell cultures (Abe et al. 2003), but stimulates osteoblast differentiation and mineralization in murine ES cell cultures through a wingless-integration 5A- (WNT5A-) dependent mechanism (Baliram, et al. 2011). In vivo, intermittently administered TSH is anabolic in both rats and mice (Sampath, et al. 2007; Sun et al. 2008). Thus, when injected at intervals as far apart as every two weeks into rats, TSH rescues the bone loss, 28 weeks following ovariectomy (Sun et al. 2008). Calcein-labeling studies are consistent with a direct bone-forming action of intermittent TSH (Sampath et al. 2007). Consistent with rodent data, Martini et al. show an increase in procollagen type I N-terminal propeptide (PINP), a marker of bone formation, validating the conclusion that a bolus dose of TSH is indeed anabolic in people (Martini, et al. 2008). Likewise, antibody-activated TSH signaling contributes to high bone formation, independent of the actions of thyroid hormone (Cho, et al. 2015).
Epidemiological studies in an increasing number of global cohorts document a tight relationship between low TSH levels, parameters of bone loss, and even fracture risk. In fact, 4.5- and 3.2-fold increases in the risk of vertebral and non-vertebral fractures, respectively, were noted at serum TSH levels <0.1 mIU/L (Bauer, et al. 2001). There was also a strong negative correlation between low serum TSH and high CTX levels, independently of thyroid hormone levels (Zofkova and Hill 2008). Greater bone loss was also seen in patients on L-thyroxine that had suppressed TSH levels than in those without such suppression (Baqi, et al. 2010; Flynn, et al. 2010; Kim, et al. 2015; La Vignera, et al. 2008). These observations were supported by the Tromsø Study, in which participants with serum TSH <2 SD had a significantly lower bone mineral density (BMD), those with TSH above 2 SD had a significantly increased BMD, whereas there was no association between TSH and BMD at normal TSH levels (Grimnes, et al. 2008). Serum levels of cathepsin K, a surrogate resorption marker, were also elevated in thyroid cancer patients on suppressive therapy (Mikosch, et al. 2008). Concordant with this, the second Nord-Trøndelag Health Study in 1995-97 (HUNT 2) found a positive correlation between serum TSH and BMD at the distal forearm (Svare, et al. 2009). In fact, it is now evident that in patients with elevated TSH levels, long-term fracture risk is related to the cumulative duration of periods with low TSH resulting from excessive thyroid hormone replacement (Abrahamsen, et al. 2015). In Odense Patient data Explorative Network Thyroid Status and Register Outcomes (OPENTHYRO), fracture risk was a function of the duration of hyperthyroidism (Abrahamsen, et al. 2014). Likewise, TSH suppression increased the risk of postoperative osteoporosis in low- and intermediate-risk thyroid cancer patients (Wang, et al. 2015c). Even hypothyroid patients requiring thyroid hormone therapy often displayed reduced BMD that correlated with low TSH levels (Christy, et al. 2014; Karimifar, et al. 2014).
The National Health and Nutrition Examination Survey (NHANES) has further shown that the odds ratio for correlations between TSH and bone mass ranged between 2 and 3.4 (Morris 2007). Elderly women with low-normal TSH levels (in the euthyroid range) have lower BMDs with or without weaker femoral structures (Ding, et al. 2016; Lee, et al. 2016). Such women also have a high vertebral fracture risk, independent of age, BMD and thyroid hormones (Mazziotti, et al. 2010). Consistent with these data, there is a positive correlation between serum TSH and BMD in women with post-menopausal osteoporosis; in one of these studies, serum TSH was associated with osteoprotection in a regression model (Acar, et al. 2016; Noh, et al. 2015; van Rijn, et al. 2014). In elderly men, lower TSH levels within the normal range were associated with an approximately 30% increase in the risk of hip fracture (Waring, et al. 2013). Finally, genetic evidence supports the epidemiologic data for a role of TSH in human bone physiology. Patients harboring the TSHRD727E polymorphism have high bone mass (Albagha, et al. 2005; Heemstra, et al. 2008; Liu, et al. 2012; van der Deure, et al. 2008).
FSH and Mechanisms of Bone Loss
FSH acts on an FSHR in the osteoclasts and directly stimulates bone resorption (Sun et al. 2006). Supporting this conclusion, mice haploinsufficient in FSHβ showed reduced bone resorption and increased bone mass, while ovarian function remained preserved (Sun et al. 2006). Several studies have now confirmed direct effects of FSH on the skeleton in rodents and humans. The exogenous administration of FSH to rats was found to augment ovariectomy-induced bone loss, and an FSH antagonist reduced bone loss after ovariectomy or FSH injection (Liu, et al. 2010a; Liu, et al. 2010b).
In women, hypergonadotropic amenorrhea (serum FSH ∼35 IU/L) was found to be associated with greater bone loss than in hypogonadotropic amenorrhea (∼8 IU/L) (Devleta, et al. 2004). Likewise, women with functional hypothalamic amenorrhea, wherein both FSH and estrogen are low, showed slight to moderate skeletal defects (Podfigurna-Stopa, et al. 2012). This is contrast to luperide, which has not been shown to prevent hypogonadal hyper-resorption, wherein the acute loss of estrogen appears not to be compensated by reduced FSH levels (Drake, et al. 2010). However, genetic evidence points to the function of FSHRs in human pathophysiology. Women harboring an activating FSHRN680S polymorphism have been shown to display lower bone mass and high resorption markers (Rendina, et al. 2010). Furthermore, digenic combinations between wild type genotype of the 3'UTR marker for the aromatase (CYP19A1) gene, the IVS4 marker of the same gene, and the bone morphogenetic protein 15 (BMP15) and FSHR genes have been described as being osteoprotective (Mendoza, et al. 2012). Together, these latter studies attest to a modulatory function for FSH, in addition to that of estrogen, in human skeletal physiology and a permissive role in the pathophysiology of post-menopausal osteoporosis.
The most impressive clinical correlations between bone loss and serum FSH levels are seen in Study of Women’s Health across the Nations (SWAN), a longitudinal cohort of 2,375 perimenopausal women. Not only was there a strong correlation between serum FSH levels and markers of bone resorption, a change in FSH levels over 4 years predicted decrements in bone mass (Sowers, et al. 2003). Data from a cohort of Chinese women showed similar trends, notably a significant association between bone loss and high serum FSH (Wu, et al. 2010; Xu, et al. 2009). Furthermore, in another cohort of southern Chinese women between 45 and 55 years, those in the highest quartile of serum FSH lost bone at a 1.3- to 2.3-fold higher rate than those in the lowest quartile (Cheung, et al. 2011). A more recent analysis of perimenopausal Chinese women aged between 45 and 50 years revealed a strong correlation between serum CTX and FSH levels (Wang, et al. 2015a). Importantly, CTX levels were greater when serum FSH levels were >40 IU/L (Wang et al. 2015a). The NHANES III cohort with women between 42 and 60 years likewise showed a strong correlation between serum FSH and femoral neck BMD (Gallagher, et al. 2010). Serum osteocalcin and CTX were also positively correlated with serum FSH, but not with estradiol in a cross-sectional study of 92 postmenopausal women (Garcia-Martin, et al. 2012). The bone turnover range of normality (BONTURNO) study group similarly showed that women with serum FSH levels >30 IU/L had significantly higher bone turnover markers than age-matched women, despite having normal menses (Adami, et al. 2008). Consistent with this, lower serum FSH levels and higher serum estrogen levels have been associated with lower rates of lumbar spine bone loss during phases of the menopausal transition (Crandall, et al. 2013).
Mechanistically, FSH increases osteoclast formation, function and survival through a distinct FSHR isoform (Robinson, et al. 2010; Sun et al. 2006; Sun, et al. 2010; Wang, et al. 2015b; Wu, et al. 2007). Failures to identify FSHRs on osteoclasts likely used primers targeted to the ovarian isoform (Allan, et al. 2010; Ritter, et al. 2008). We very consistently find FSHR in human CD14+ cells and osteoclasts using nested primers and sequencing to verify the specificity of the reaction, and amplifying regions that contain an intron to avoid the pitfall of genomic DNA contamination (Robinson et al. 2010; Tourkova, et al. 2015). Cellular responsiveness to FSH also appears to be determined by the level of FSH glycosylation (Meher, et al. 2015), with the prediction that the fully glycosylated isoform is more active on the bone FSHR. With that said, FSHR expression in bone has recently been firmly established through in vivo imaging of FSH binding in live mice (Feng et al. 2017). A small near infrared fluorophore CH1055 conjugated to FSH was injected into live mice, followed by capture of infrared signals upon binding to its receptor in ovaries, testes and bone in vivo (Feng et al. 2017).
The osteoclastogenic response to FSH was interestingly abolished in mice lacking immunoreceptor tyrosine-based activation motif (Itam) adapter signaling molecules (Wu et al. 2007). This latter study suggests an interaction between FSH and immune receptor complexes, although the significance of this remains unclear. In a separate study, FSH enhanced expression of receptor activator of nuclear factor kappa B (RANK) (Cannon, et al. 2011). In addition, FSH indirectly stimulated osteoclast formation by releasing osteoclastogenic cytokines, namely interleukin 1β (IL1B), TNFα and interleukin 6 (IL6) in proportion to the surface expression of FSHRs (Cannon, et al. 2010; Iqbal, et al. 2006). In a study of 36 women between the ages of 20 and 50, serum FSH levels correlated with circulating cytokine concentrations (Cannon et al. 2010; Gertz, et al. 2010). Gourlay et al. in contrast failed to show a relationship between bone mass and FSH or, indeed, estrogen (Gourlay, et al. 2011). Interestingly, the same authors documented an independent correlation between FSH and lean mass (Gourlay, et al. 2012).
Despite accumulating evidence for separate actions of FSH, it has been difficult to tease out the action of FSH from that of estrogen in vivo, as FSH releases estrogen, and the actions of FSH and estrogen on the osteoclast are opposed. The injection of FSH into mice with intact ovaries (Ritter et al. 2008), or its transgenic over-expression (Allan et al. 2010), even in hpg mice, is therefore unlikely to reveal pro-resorptive actions of FSH. This is because direct effects of FSH on the osteoclast will invariably be masked by the anti-resorptive and anabolic actions of the ovarian estrogen so released in response to FSH. However, there is evidence that women with low FSH levels undergo less bone loss (Devleta et al. 2004), and that the effectiveness of estrogen therapy is related to the degree of FSH suppression (Kawai, et al. 2004).
Rapid and profuse bone loss begins three years prior to the last menstrual period, when serum estrogen is relatively normal and FSH levels are rising (Randolph, et al. 2003). This is when the rate of bone loss is maximal, and therefore cannot be attributed to changes in serum estrogen (Randolph et al. 2003; Sowers et al. 2003). To address the importance of FSH elevations in this period, Lukefahr and coworkers examined bone loss using a unique rat model of the perimenopausal transition (Lukefahr, et al. 2012). This model, in which the ovotoxin 4-vinylcyclohexene diepoxide (VCD) is administered to rats, was characterized by a prolonged estrogen-replete period when serum FSH levels were elevated. Longitudinal measurements revealed that significant decreases in BMD (5–13%) occurred during periods of increased FSH and decreased inhibins (Lukefahr et al. 2012).
To leverage this selective increase in FSH early in the menopausal transition, an antibody to a 13-amino-acid-long peptide sequence within the receptor-binding domain of the FSH β-subunit was generated (Zhu, et al. 2012a; Zhu, et al. 2012b). The FSH antibody bound FSH specifically and blocked its action on osteoclast formation in vitro. When injected into ovariectomized mice, the FSH antibody attenuated bone loss not only by inhibiting bone resorption, but also by stimulating bone formation, an action we had failed to detect in the knock out models (Sun et al. 2006; Zhu et al. 2012a; Zhu et al. 2012b). Notably, stromal cells isolated from mice treated with the FSH antibody showed greater osteoblast precursor colony counts, similarly to stromal cells isolated from Fshr−/− mice (Zhu et al. 2012a). This suggested that FSH negatively regulates osteoblast differentiation via signaling-efficient FSHRs present on mesenchymal stem cells (Zhu et al. 2012a). There is recent direct evidence for FSH action on osteoblast precursors, albeit in the opposite direction (Su, et al. 2017). Overall, the data prompt the future development of a novel FSH-blocking agent as a means of uncoupling bone formation and bone resorption to a therapeutic advantage in people. An interesting alternative strategy, for which a proof-of-principle study is available, is the use of an FSHβ vaccine. It has been shown that immunizing ovariectomized rats with the GST-FSHβ antigen significantly prevents trabecular bone loss and increases bone strength (Geng, et al. 2013).
GH in Skeletal Maturation
Growth hormone (GH) has for long been known to play a key role in chondrocyte and bone cell function, and thereby to modulate skeletal growth and modeling. While a GHR is expressed in osteoblasts, GH exerts appears to exert an action via its release of insulin-like growth factors (IGFs). IGF1, the predominant growth factor, is synthesized mainly in the liver and 80% of the circulating form is bound to IGF binding protein-3 (IGFBP3) and the acid labile subunit (ALS). Growth retardation and osteoporosis are phenotypic hallmarks in Ghr−/− mice (De Jesus, et al. 2009) and dwarfism in Laron syndrome, which is due to loss-of-function mutations in the GHR gene, is associated with osteoporosis (Laron 1999; Laron, et al. 1999). However, the Ghr−/− phenotype is compensated by the over-expression of IGF1 and mice lacking both liver IGF1 (LID) and ALS, with depleted serum IGF1, show reduced bone growth and bone strength in the face of high circulating GH levels (Yakar, et al. 2002). While these results suggest that the skeletal effects of GH require IGF1, there is equally compelling evidence that GH can act independently of IGF. In ovariectomized LID mice, for example, GH reverses osteopenia (Fritton, et al. 2010). Furthermore, GH replacement reverses the increased adiposity in hypophysectomized rats, while IGF1 replacement does not (Menagh, et al. 2010). Together, the latter findings not only point to a direct action of GH on bone, but also extend GH actions to the control of adiposity (see below).
Pituitary Hormones and Intergenerational Calcium Transfer
A major function of the skeleton is to serve as a source for calcium ions that would mineralize the fetal skeleton during pregnancy and allow for bone growth and modeling during lactation. Both phases are characterized by excessive maternal bone resorption that has been thought to result from a combination of hypoestrogenemia and parathyroid hormone-related protein (PTHrP) production by the breast tissue (Wysolmerski 2002). Despite the loss of the maternal skeleton, with noted reductions in BMD in the region of 1–2% per month, this bone loss is near-completely reversed upon weaning (Sowers, et al. 1995). The mechanism of the latter effect is unclear; however, ineffective skeletal restoration, for example over multiple pregnancies, can lead to pregnancy- and lactation-associated osteoporosis.
Plasma OXT levels peak during late pregnancy and lactation. In addition to its action on milk ejection, uterine contraction, social behavior and feeding, OXT acts on a GPCR present in abundance on osteoblasts, osteoclasts and their precursors (Argiolas, et al. 1988; Colucci, et al. 2002; Copland, et al. 1999; Elabd, et al. 2008; Insel and Harbaugh 1989; Mantella, et al. 2003; Nishimori, et al. 1996; Sclafani, et al. 2007; Tamma, et al. 2009; Young, et al. 1998). Bone marrow cells also synthesize OXT, suggesting the existence of autocrine and paracrine interactions (Colaianni, et al. 2011; Tamma et al. 2009).
OXT has been shown to stimulate osteoblast differentiation and bone formation in mice (Colucci et al. 2002; Elabd et al. 2008; Tamma et al. 2009), and Oxt−/− and Oxtr−/− mice, importantly normally lactating heterozygotes, display severe osteoporosis due to a bone-forming defect (Tamma et al. 2009). Systemic OXT injections into wild type rodents stimulate bone formation, increase bone mass, and enhance osseointegration of titanium implants, again attesting to the skeletal anabolic action of OXT (Elabd, et al. 2007; Tamma et al. 2009; Wang, et al. 2016). These pro-osteoblastic actions are mediated, at least in part, via the nuclear localization of the OXTR following its activation by OXT and, unlike actions on social behavior and feeding, are not mediated centrally (Di Benedetto, et al. 2014; Tamma et al. 2009). Thus, short-term intracerebroventricular OXT does not affect bone turnover markers.
In contrast to the potent osteoblastic actions of OXT, its overall effects on bone resorption in vivo appear minimal. OXT stimulates osteoclastogenesis, but inhibits the activity of mature osteoclasts (Tamma et al. 2009). While these pro-osteoclastogenic actions may contribute to intergenerational calcium transfer, the anabolic effect is likely to facilitate restoration of the maternal skeleton. Testifying to this, Oxt−/− pups show hypomineralized skeletons, and Oxt−/− moms display reduced bone formation markers (Liu, et al. 2009).
Estrogen positively regulates osteoblastic OXT production and OXTR expression, and the respective actions are synergistic through a local feed-forward loop (Colaianni, et al. 2012; Zallone 2006). In postmenopausal women, serum OXT correlates strongly with BMD, particularly at the hip, in the 6-year-long prospective Opsumit Users (OPUS) study (Breuil, et al. 2014). Likewise, in a cross-sectional study in women aged between 18 and 45 years, plasma OXT levels were correlated with spine and hip BMD and buckling ratio at the hip (Schorr, et al. 2017). Low serum OXT levels, in contrast, are associated with severe osteoporosis, independently of estrogen (Breuil, et al. 2011). In separate studies, the low nocturnal OXT secretion in amenorrheic athletes is associated with site-dependent microarchitectural deterioration (Lawson, et al. 2013). However, in men, the Montceau les Mines Osteoporosis (MINOS) study confirms a lack of association between serum OXT and BMD, bone turnover rate or prevalent fracture (Breuil, et al. 2015). Likewise, in mice and rabbits, OXT reverses ovariectomy-, but not orchiectomy-induced bone loss (Beranger, et al. 2015; Beranger, et al. 2014; Qiu, et al. 2015). The findings together emphasize possible sex differences that might arise from the regulation of OXT action by estrogen (Colaianni et al. 2012).
Excessive secretion of prolactin (PRL) is also associated with accelerated bone turnover and osteoporosis in hyperprolactinemic states (Naylor, et al. 2000). Antagonism of PRL by bromocriptine, a dopamine agonist, reverses the bone loss (Lotinun, et al. 2003). It has also long been speculated that, in addition to its permissive role during lactation and suppression folliculogenesis and libido, PRL increases calcium bioavailability for both milk production and fetal skeletalogenesis by promoting skeletal mobilization (Lotinun, et al. 1998). This pro-resorptive action has traditionally been thought to arise due to the accompanying hypoestrogenemia (Meaney, et al. 2004). However, osteoblasts express PRL receptors (PRLRs) (Coss, et al. 2000), suggesting that reduced osteoblastic bone formation may also play a significant role in the bone loss. The pattern of this bone loss is also distinct from that of ovariectomized rodents, where there is evidence for increased bone resorption and formation, with resorption exceeding formation. In contrast, in PRL-exposed rats there is a decoupling between the two processes with increases in bone resorption that are not accompanied by reduced bone formation (Seriwatanachai, et al. 2008b). Consistent with this phenotype, PRL decreases osteoblast differentiation markers, in part, through the phosphoinositide 3-kinase (PI3K) signaling pathway (Seriwatanachai, et al. 2008a; Seriwatanachai et al. 2008b). Osteoclasts themselves do not possess PRLRs and PRL displays indirect pro-resorptive effects by increasing the RANK ligand/osteoprotegerin (RANKL/OPG) ratio (Coss et al. 2000; Seriwatanachai et al. 2008b).
There is also evidence that the effect of PRL on bone depends on the biological maturity of the organism. In infant rats, PRL causes net bone gain and increased osteocalcin expression (Krishnamra and Seemoung 1996). Likewise, in fetal human osteoblasts, PRL decreases the RANKL/OPG ratio (Seriwatanachai et al. 2008a). These studies point to a role for PRL in bone growth and mineralization in utero, while accelerating bone resorption from the maternal skeleton for intergenerational calcium transfer. Further insight is needed to clarify the role of PRL in bone metabolism and determine the cellular pathways.
AVP and Bone Loss in Hyponatremia
There are abundant receptors for arginine vasopressin (AVP), namely AVPR1α and AVPR2, coupled to extracellular signal-regulated kinase (ERK) activation, on both osteoblasts and osteoclasts (Tamma, et al. 2013). However, opposed to the anabolic action of OXT (Tamma et al. 2009), AVP reduces osteoblast differentiation and bone formation and enhances osteoclastogenesis and bone resorption (Tamma et al. 2013). In loss-of-function studies, the exposure of osteoblast precursors to AVPR1α or AVPR2 antagonists, namely relcovaptan (SR49059) or [Adamantaneacetyl1, o-Et-d-Tyr2, Val4, Aminobutyryl6, Arg8,9]vasopressin (ADAM), causes increased osteoblastogenesis, effects that are phenocopied in Avpr1α−/− mice. Osteoclast formation and bone resorption are both reduced in these mice (Tamma et al. 2013), in sum, causing a profound increase of bone mass (Tamma et al. 2013). The anabolic and anti-anabolic actions, respectively, of OXT and AVP interact, so that the increased bone mass in Avpr1α−/− mice is reduced in compound Avpr1α−/−:Oxtr−/− mutants (Sun, et al. 2016). In fact, AVP and OXT can share receptors to a limited extent (Sun et al. 2016). While the OXTR is not indispensable for AVP action in inhibiting osteoblastogenesis, AVP-stimulated gene expression is inhibited when the OXTR is deleted in Avpr1α−/− cells. In contrast, OXT does not interact with AVPRs in vivo in a model of lactation-induced bone loss, where OXT levels are high (Sun et al. 2016).
Together, the data establish a primary role for AVP signaling in bone mass regulation. They also shed light on a long-standing dogma on the mechanism(s) of the osteoporosis that accompanies chronic hyponatremic states (Barsony, et al. 2011; Gankam Kengne, et al. 2008; Hoorn, et al. 2011; Kinsella, et al. 2010; Renneboog, et al. 2006; Sandhu, et al. 2009; Verbalis, et al. 2010). It is quite possible that, in a case report, the documented bone loss in a patient with syndrome of inappropriate antidiuretic hormone (SIADH) had arisen from a 30-fold elevation in serum AVP, although the accompanying high aldosterone could be an additional culprit (Balla, et al. 1981; Carbone, et al. 2008; Chhokar, et al. 2004; Sejling, et al. 2012). Furthermore, while AVPR2 is also expressed osteoblasts and osteoclasts, it is unlikely from our results that highly selective aquaretic AVPR2 inhibitors, such as tolvaptan, could, in fact, themselves offer osteoprotection (Hori 2013; Sun et al. 2016). AVPR1α therefore appears to be the major functional skeletal receptor for AVP.
Pituitary Hormones and Body Composition
An acute need for anti-obesity agents arises not only from the increasing population of obese individuals worldwide, currently around 600 million, but also from the relatively small armamentarium of approved anti-obesity agents that are limited by poor efficacy and unacceptable side effects. Furthermore, in women, FSH secretion begins to increase during the perimenopausal transition, preceding declines in estrogen by about 2 to 3 years (Seifert-Klauss, et al. 2012; Sowers et al. 2003). During this period, and concurrent with the sharp decline in BMD, there is the onset of increasing visceral and bone marrow adiposity and a decrease in lean mass (Thurston, et al. 2009; Wildman, et al. 2012). This clinical phenotype is associated with disrupted energy metabolism and decreased physical activity (Thurston et al. 2009). Considering that FSHRs are present on bone and fat tissue (Cui, et al. 2012; Feng et al. 2017; Liu et al. 2017; Liu et al. 2015; Sun et al. 2006), a question has arisen whether a single agent can be used to treat two aging-related medical conditions – osteoporosis and obesity – simultaneously.
We therefore utilized our polyclonal antibody to FSHβ to examine the effect of FSH blockade in mice pair-fed on a high-fat diet, ovariectomized and sham operated mice, and mice on a normal chow, but allowed to eat ad libitum (Liu et al. 2017). In all instances, the FSH antibody prevented the development of visceral and subcutaneous obesity and induced energy-producing ‘beige’ adipocytes. This beiging phenotype was evident from immunolabeling for uncoupling protein 1 (UCP1), measuring the expression of Ucp1 and other brown adipose tissue (BAT) genes in white adipose tissue, and examining UCP1 expression in vivo in live UCP1 reporter, Thermo mice (Liu et al. 2017). Furthermore, the FSH antibody enhanced mitochondrial density in the photo-activatable mitochondria (PhAM) mouse and enhanced basal energy expenditure and oxygen consumption, independently of increased activity. Studies with a corresponding monoclonal FSH antibody to human FSH recapitulated the data, as did mice haploinsufficient in the Fshr. Importantly, the FSH antibody failed to prevent obesity in Fshr+/− mice, testifying to its action via the FSH axis in vivo. Finally, there was an important, yet unexplained increase in lean mass in all groups treated with the FSH antibody. This phenotype is concordant with a strong association between reduced lean mass and high FSH levels in post-menopausal women (Gourlay et al. 2012).
TSHRs are also present in adipose tissue, although their precise function in physiology remains to be established. There is evidence from TSHR signaling-deficient hyt/hyt mice that TSHRs may be involved in inducing lipolysis in white adipose tissue, especially in mice in a hypothyroid state (Endo and Kobayashi 2012). Similarly to the FSHR expression (Liu et al. 2017), TSHRs were induced upon differentiation of 3T3.L1 pre-adipocytes, and TSHR knockdown blocked adipocyte differentiation (Lu, et al. 2012). Tshr expression was also higher in visceral adipose tissues from mice fed on a high-fat diet. In humans, TSHR expression in subcutaneous adipose tissue correlated with body mass index (BMI) (Lu et al. 2012).
Epidemiologic studies support the notion that TSH is associated with an obesity phenotype. In a cross-sectional study of 194 healthy euthyroid postmenopausal women, BMI had a positive independent association with serum TSH levels (Lambrinoudaki, et al. 2015). Serum TSH also correlated with BMI in a cohort of 800 hospitalized patients with BMIs of ≥30 kg/m2 (Betry, et al. 2015). Furthermore, in 60 euthyroid subjects, TSH correlated with BMI and visceral and subcutaneous adipose tissue volumes (Muscogiuri, et al. 2013). Serum TSH has also been noted as being a predictor for obesity in euthyroid females. Notably, body weight, BMI, waist circumference and body fat were all found to be increased in women with serum TSH >2.5 mIU/L compared to those with TSH ≤2.5 mIU/L (Zhang, et al. 2012). Moreover, in patients with subclinical hypothyroidism who had high pretreatment serum TSH levels, but normal thyroid hormones, a significant decrease in visceral adipose tissue volume was noted following levothyroxine treatment (Akbaba, et al. 2016).
With that said, several studies have failed to document correlations or shown negative correlations between serum TSH and obesity indices. Notably, a population-based German cohort consisting of over 2000 adults aged between 20 and 79 years showed no association between serum TSH levels and visceral adipose tissue (Witte, et al. 2017). In a different cohort of over 4800 patients, BMI was inversely related to higher TSH levels in patients who were classified as BAT-positive upon F-18-fluordesoxyglucose positron emission tomography-computer tomography (FDG-PET/CT) (Brendle, et al. 2017).
In addition to FSH and TSH, there is also a strong and well-documented relationship between OXT and body composition. Injection of OXT reduced body weight in mice fed both on high-fat diet or normal chow, body fat in ovariectomized mice, and bone marrow fat in glucocorticoid-treated rabbits (Beranger et al. 2014; Li, et al. 2016; Maejima, et al. 2017; Qiu et al. 2015). Reduced OXT expression, in contrast, underscores hyperphagic obesity of single-minded 1 (Sim1) haploinsufficient mice, which are also cured upon OXT injection (Tolson, et al. 2010). A cross-sectional study of women aged between 18 and 45 years further documented highest plasma OXT levels in those who were overweight or obese, with strong correlations between OXT levels and BMI, and total, visceral, and subcutaneous fat (Schorr et al. 2017).
However, in contrast to the peripheral action of FSH and TSH through adipose tissue GPCRs, OXT appears to act on the central nervous system to regulate body composition via a primary action on feeding. Several lines of evidence attest to this. First, unlike bone cells, there are no OXTRs yet identified in fat tissue. Second, chronic central OXT infusion in high-fat-diet-induced obese rats resulted in decreased body weight, increased adipose tissue lipolysis, increased fatty acid β-oxidation, and reduced glucose intolerance and insulin resistance (Deblon, et al. 2011). Third, feeding stimulated and starvation reduced OXT secretion from the pituitary (Kublaoui, et al. 2008; Zhang and Cai 2011). Fourth, patients affected by SIM1 gene mutation and by Prader–Willi syndrome, in who hyperphagia and obesity are characteristic clinical features, displayed reduced numbers and size of OXT-ergic neurons in paraventricular nuclei (Holder, et al. 2000; Swaab, et al. 1995). These findings suggest that the prominent effects of OXT on body composition are mediated centrally through an effect on feeding, although there is some evidence for a peripheral action. For example, the late-onset obesity in Oxtr−/− mice is independently of daily intake of chow (Takayanagi, et al. 2008). However, OXT infusion by subcutaneously implanted osmotic minipumps in diet-induced obese mice reduced food intake (Maejima, et al. 2011; Morton, et al. 2012). Intraperitoneal OXT injection likewise induced c-Fos expression in the hypothalamus (Maejima et al. 2011). These findings together suggest that peripherally administered OXT could cross the blood brain barrier and vice versa. Cell-specific conditional deletion of OXTRs in the periphery versus centrally in OXT-ergic neurons should provide firm conclusions regarding the primary site of action of OXT.
Ubiquity of ‘Pituitary’ Hormone Expression
Not only have receptors been identified on tissues other than what are considered primary endocrine targets, ‘pituitary’ hormones themselves are produced in non–pituitary locations. Among the most exciting revelations has been the clear documentation of GH production in various compartments of the eye, particularly in retinal ganglion cells(Baudet, et al. 2003; Harvey and Aramburo 2017; Harvey, et al. 2004; Martinez-Moreno, et al. 2018). Here, they act on GHRs to regulate retinal development, and specifically the processes of neurite outgrowth, retinal vascularization, and neuronal cell proliferation and apoptosis (Harvey and Aramburo 2017; Martinez-Moreno et al. 2018). Transgenic GH overexpression has been associated with increased axial length of the eye, whereas siRNA knockdown reduces axonal growth(Grimbly, et al. 2009; Martin, et al. 2011). Additionally, GH is expressed in lymphoid tissues, including thymus and spleen, as well as in both the male and female reproductive tracts (Hull and Harvey 2014). However, apart from effects on steroidogenesis and ovarian cell proliferation, the mechanism of these extra–pituitary actions is relatively unclear.
Extra–pituitary sources of TSH have long been known (Blalock 2005; Smith, et al. 1983a). In parallel with the pituitary–thyroid endocrine axis, there is additional TSH–related circuitry that functions beyond the thyroid to involve, for example, the immune system. Immune cells, mainly macrophages, produce a novel TSH–βv (Baliram, et al. 2013; Baliram, et al. 2016; Vincent et al. 2009a). In the mouse, the TSH–β coding region is located in segments of exons 4 and 5, while exon 4 is missing in mouse TSH–βv. In contrast, the human TSH gene contains three exonic sequences, but exon 2 is missing in the human TSH–βv. Molecular docking and cell–based assays show that TSH–β and TSH–βv bind to and signal through the TSHR with similar binding affinities (Baliram et al. 2013; Baliram et al. 2016). However, while thyroid hormones suppress the production of pituitary TSH, macrophage–derived TSH–βv is enhanced. Locally produced TSH–βv in response to hyperthyroidism may, in fact, offer some limited protection to the skeleton (Baliram et al. 2012), but this assumption is speculative. However, interestingly, TSH–βv is also synthesized by mouse and human pituitary (Schaefer and Klein 2009; Vincent, et al. 2009b). Lymphocytes are also known to express TSH (Harbour, et al. 1989; Smith, et al. 1983b), but there is no evidence yet for bone or marrow cell production of FSH, although co-production of TSH–β and FSH is noted in CD11b+ cells from mouse thyroid (Klein and Wang 2004).
We have shown that Oxt, apart from its neurohypophyseal origin, is produced in abundance by both human and murine osteoblasts (Colaianni et al. 2011). Production of osteoblast Oxt is under the control of estrogen, which acts by activating the MAP kinase Erk (Colaianni et al. 2011; Colaianni et al. 2012). This non-genomic mechanism of estrogen action is in contrast to its genomic control of Oxtr expression (Tamma et al. 2009). We surmise that there is a local feed–forward loop in bone marrow through which the Oxt so produced from osteoblasts in response to estrogen acts upon its receptor to exert a potent anabolic action. We also show that the absence of Oxtrs in osteoblasts derived from Oxtr−/− mice or the attenuation of Oxtr expression in silenced cells inhibits estrogen–induced osteoblast differentiation, transcription factor up-regulation, and/or Oxt production in vitro. In vivo, Oxtr−/− and Col2.3-Cre+:Oxtrfl/fl mice display a bone formation defect, and fail to display increases in trabecular bone volume, cortical thickness, and bone formation in response to estrogen (Colaianni et al. 2012). Physiologically, therefore, feed–forward Oxt release in bone marrow by a rising estrogen concentration may facilitate rapid skeletal recovery during the latter phases of lactation.
Conclusion
The direct regulation of body composition, importantly bone mass and adipose tissue, by hormones from pituitary gland helps explain some of the inconsistencies of older models that assumed monogamous pituitary signaling through single targets. However, this latter assumption of singularity cannot be explained by highly distributed functions of primitive pituitary hormones in lower organisms. Non-traditional and important direct actions have thus emerged, which relate to effects of both anterior and posterior pituitary hormones on bone mass, body fat, and energy homeostasis. Interestingly, these newly described signaling pathways appear not to have identical mechanisms to traditional endocrine targets, so that, for example, the FSHR in bone and fat tissue couples with a Gi protein as opposed to traditional FSHR-Gs coupling in the ovarian follicular cell. There is also increasing evidence for ligands, receptors and their splice variants to be co-expressed in the same tissue, in essence, highlighting yet uncharacterized paracrine and autocrine control mechanisms. Newly discovered targets of these otherwise ‘old’ hormones also provide unique opportunities for the therapy of diseases that we can now attribute to changes in the levels of circulating pituitary hormones. Thus, we posit that there is a pituitary–bone and pituitary–adipose axis that, when perturbed, causes diseases, such as obesity and osteoporosis, but can be potentially utilized to target novel therapeutics for use in people.
Acknowledgments
Mone Zaidi, Li Sun, Terry F. Davies, Harry C. Blair and Clifford J. Rosen are supported by grants from the National Institutes of Health. Alberta Zallone is supported by the Ministry of Education, Italy. Maria I. New is supported by the Children’s Hormone Research Foundation, New York, USA.
Footnotes
Disclosures
Mone Zaidi is a named inventor of an issued US patent related to osteoclastic bone resorption filed by the Icahn School of Medicine at Mount Sinai (ISMMS). In the event the issued patent is licensed, he would be entitled to a share of any proceeds ISMMS receives from the licensee. All other authors have nothing to disclose.
References
- Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J, Eldeiry L, Rajendren G, Blair HC, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- Abrahamsen B, Jorgensen HL, Laulund AS, Nybo M, Bauer DC, Brix TH, Hegedus L. The excess risk of major osteoporotic fractures in hypothyroidism is driven by cumulative hyperthyroid as opposed to hypothyroid time: an observational register-based time-resolved cohort analysis. Journal of Bone and Mineral Research. 2015;30:898–905. doi: 10.1002/jbmr.2416. [DOI] [PubMed] [Google Scholar]
- Abrahamsen B, Jorgensen HL, Laulund AS, Nybo M, Brix TH, Hegedus L. Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures-the OPENTHYRO register cohort. Journal of Bone and Mineral Research. 2014;29:2040–2050. doi: 10.1002/jbmr.2244. [DOI] [PubMed] [Google Scholar]
- Acar B, Ozay AC, Ozay OE, Okyay E, Sisman AR, Ozaksoy D. Evaluation of thyroid function status among postmenopausal women with and without osteoporosis. International Journal of Gynecology & Obstetrics. 2016;134:53–57. doi: 10.1016/j.ijgo.2015.11.025. [DOI] [PubMed] [Google Scholar]
- Adami S, Bianchi G, Brandi ML, Giannini S, Ortolani S, DiMunno O, Frediani B, Rossini M. group Bs. Determinants of bone turnover markers in healthy premenopausal women. Calcified Tissue International. 2008;82:341–347. doi: 10.1007/s00223-008-9126-5. [DOI] [PubMed] [Google Scholar]
- Akbaba G, Berker D, Isik S, Tuna MM, Koparal S, Vural M, Yilmaz FM, Topcuoglu C, Guler S. Changes in the before and after thyroxine treatment levels of adipose tissue, leptin, and resistin in subclinical hypothyroid patients. Wien Klin Wochenschr. 2016;128:579–585. doi: 10.1007/s00508-015-0865-9. [DOI] [PubMed] [Google Scholar]
- Albagha OME, Natarajan R, Reid DM, Ralston SH. The D727E polymorphism of the human thyroid stimulating hormone receptor is associated with bone mineral density and bone loss in women from the UK. Journal of Bone and Mineral Research. 2005;20:S341–S341. [Google Scholar]
- Allan CM, Kalak R, Dunstan CR, McTavish KJ, Zhou H, Handelsman DJ, Seibel MJ. Follicle-stimulating hormone increases bone mass in female mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22629–22634. doi: 10.1073/pnas.1012141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiolas A, Collu M, Gessa GL, Melis MR, Serra G. The oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin inhibits male copulatory behaviour in rats. European Journal of Pharmacology. 1988;149:389–392. doi: 10.1016/0014-2999(88)90675-9. [DOI] [PubMed] [Google Scholar]
- Baliram R, Chow A, Huber AK, Collier L, Ali MR, Morshed SA, Latif R, Teixeira A, Merad M, Liu L, et al. Thyroid and bone: macrophage-derived TSH-beta splice variant increases murine osteoblastogenesis. Endocrinology. 2013;154:4919–4926. doi: 10.1210/en.2012-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliram R, Latif R, Berkowitz J, Frid S, Colaianni G, Sun L, Zaidi M, Davies TF. Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16277–16282. doi: 10.1073/pnas.1110286108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliram R, Latif R, Morshed SA, Zaidi M, Davies TF. T3 Regulates a Human Macrophage-Derived TSH-beta Splice Variant: Implications for Human Bone Biology. Endocrinology. 2016;157:3658–3667. doi: 10.1210/en.2015-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliram R, Sun L, Cao J, Li J, Latif R, Huber AK, Yuen T, Blair HC, Zaidi M, Davies TF. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. Journal of Clinical Investigation. 2012;122:3737–3741. doi: 10.1172/JCI63948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T, Nagy K, Tarjan E, Renczes G, Spat A. Effect of reduced extracellular sodium concentration on the function of adrenal zona glomerulosa: studies in conscious rats. Journal of Endocrinology. 1981;89:411–416. doi: 10.1677/joe.0.0890411. [DOI] [PubMed] [Google Scholar]
- Baqi L, Payer J, Killinger Z, Susienkova K, Jackuliak P, Cierny D, Langer P. The level of TSH appeared favourable in maintaining bone mineral density in postmenopausal women. Endocrine Regulations. 2010;44:9–15. doi: 10.4149/endo_2010_01_9. [DOI] [PubMed] [Google Scholar]
- Barsony J, Sugimura Y, Verbalis JG. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. Journal of Biological Chemistry. 2011;286:10864–10875. doi: 10.1074/jbc.M110.155002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudet ML, Sanders EJ, Harvey S. Retinal growth hormone in the chick embryo. Endocrinology. 2003;144:5459–5468. doi: 10.1210/en.2003-0651. [DOI] [PubMed] [Google Scholar]
- Bauer DC, Ettinger B, Nevitt MC, Stone KL Study of Osteoporotic Fractures Research G. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Annals of Internal Medicine. 2001;134:561–568. doi: 10.7326/0003-4819-134-7-200104030-00009. [DOI] [PubMed] [Google Scholar]
- Beranger GE, Djedaini M, Battaglia S, Roux CH, Scheideler M, Heymann D, Amri EZ, Pisani DF. Oxytocin reverses osteoporosis in a sex-dependent manner. Frontiers in Endocrinology. 2015;6:81. doi: 10.3389/fendo.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranger GE, Pisani DF, Castel J, Djedaini M, Battaglia S, Amiaud J, Boukhechba F, Ailhaud G, Michiels JF, Heymann D, et al. Oxytocin reverses ovariectomy-induced osteopenia and body fat gain. Endocrinology. 2014;155:1340–1352. doi: 10.1210/en.2013-1688. [DOI] [PubMed] [Google Scholar]
- Betry C, Challan-Belval MA, Bernard A, Charrie A, Drai J, Laville M, Thivolet C, Disse E. Increased TSH in obesity: Evidence for a BMI-independent association with leptin. Diabetes Metab. 2015;41:248–251. doi: 10.1016/j.diabet.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Blair HC, Robinson LJ, Sun L, Isales C, Davies TF, Zaidi M. Skeletal receptors for steroid-family regulating glycoprotein hormones: A multilevel, integrated physiological control system. Annals of the New York Academy of Sciences. 2011;1240:26–31. doi: 10.1111/j.1749-6632.2011.06287.x. [DOI] [PubMed] [Google Scholar]
- Blalock JE. The immune system as the sixth sense. J Intern Med. 2005;257:126–138. doi: 10.1111/j.1365-2796.2004.01441.x. [DOI] [PubMed] [Google Scholar]
- Bogerd J, Granneman JC, Schulz RW, Vischer HF. Fish FSH receptors bind LH: how to make the human FSH receptor to be more fishy? General and Comparative Endocrinology. 2005;142:34–43. doi: 10.1016/j.ygcen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Brendle C, Werner MK, Schmadl M, la Fougere C, Nikolaou K, Stefan N, Pfannenberg C. Correlation of Brown Adipose Tissue with Other Body Fat Compartments and Patient Characteristics: A Retrospective Analysis in a Large Patient Cohort Using PET/CT. Academic Radiology. 2017 doi: 10.1016/j.acra.2017.09.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Breuil V, Amri EZ, Panaia-Ferrari P, Testa J, Elabd C, Albert-Sabonnadiere C, Roux CH, Ailhaud G, Dani C, Carle GF, et al. Oxytocin and bone remodelling: relationships with neuropituitary hormones, bone status and body composition. Joint Bone Spine. 2011;78:611–615. doi: 10.1016/j.jbspin.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Breuil V, Fontas E, Chapurlat R, Panaia-Ferrari P, Yahia HB, Faure S, Euller-Ziegler L, Amri EZ, Szulc P. Oxytocin and bone status in men: analysis of the MINOS cohort. Osteoporosis International. 2015;26:2877–2882. doi: 10.1007/s00198-015-3201-3. [DOI] [PubMed] [Google Scholar]
- Breuil V, Panaia-Ferrari P, Fontas E, Roux C, Kolta S, Eastell R, Ben Yahia H, Faure S, Gossiel F, Benhamou CL, et al. Oxytocin, a new determinant of bone mineral density in post-menopausal women: analysis of the OPUS cohort. Journal of Clinical Endocrinology and Metabolism. 2014;99:E634–641. doi: 10.1210/jc.2013-4126. [DOI] [PubMed] [Google Scholar]
- Britto JM, Fenton AJ, Holloway WR, Nicholson GC. Osteoblasts mediate thyroid hormone stimulation of osteoclastic bone resorption. Endocrinology. 1994;134:169–176. doi: 10.1210/endo.134.1.8275930. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Cortez-Cooper M, Meaders E, Stallings J, Haddow S, Kraj B, Sloan G, Mulloy A. Follicle-stimulating hormone, interleukin-1, and bone density in adult women. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2010;298:R790–798. doi: 10.1152/ajpregu.00728.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JG, Kraj B, Sloan G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine. 2011;53:141–144. doi: 10.1016/j.cyto.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone LD, Cross JD, Raza SH, Bush AJ, Sepanski RJ, Dhawan S, Khan BQ, Gupta M, Ahmad K, Khouzam RN, et al. Fracture risk in men with congestive heart failure risk reduction with spironolactone. Journal of the American College of Cardiology. 2008;52:135–138. doi: 10.1016/j.jacc.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Cheung E, Tsang S, Bow C, Soong C, Yeung S, Loong C, Cheung CL, Kan A, Lo S, Tam S, et al. Bone loss during menopausal transition among southern Chinese women. Maturitas. 2011;69:50–56. doi: 10.1016/j.maturitas.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, Smith RA, Gerling IC, Weber KT. Loss of bone minerals and strength in rats with aldosteronism. American Journal of Physiology - Heart and Circulatory Physiology. 2004;287:H2023–2026. doi: 10.1152/ajpheart.00477.2004. [DOI] [PubMed] [Google Scholar]
- Cho SW, Bae JH, Noh GW, Kim YA, Moon MK, Park KU, Song J, Yi KH, Park do J, Chung JK, et al. The Presence of Thyroid-Stimulation Blocking Antibody Prevents High Bone Turnover in Untreated Premenopausal Patients with Graves' Disease. PLoS One. 2015;10:e0144599. doi: 10.1371/journal.pone.0144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy AL, D'Souza V, Babu RP, Takodara S, Manjrekar P, Hegde A, Rukmini MS. Utility of C-terminal Telopeptide in Evaluating Levothyroxine Replacement Therapy-Induced Bone Loss. Biomarker Insights. 2014;9:1–6. doi: 10.4137/BMI.S13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G, Di Benedetto A, Zhu LL, Tamma R, Li J, Greco G, Peng Y, Dell'Endice S, Zhu G, Cuscito C, et al. Regulated production of the pituitary hormone oxytocin from murine and human osteoblasts. Biochemical and Biophysical Research Communications. 2011;411:512–515. doi: 10.1016/j.bbrc.2011.06.158. [DOI] [PubMed] [Google Scholar]
- Colaianni G, Sun L, Di Benedetto A, Tamma R, Zhu LL, Cao J, Grano M, Yuen T, Colucci S, Cuscito C, et al. Bone marrow oxytocin mediates the anabolic action of estrogen on the skeleton. Journal of Biological Chemistry. 2012;287:29159–29167. doi: 10.1074/jbc.M112.365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci S, Colaianni G, Mori G, Grano M, Zallone A. Human osteoclasts express oxytocin receptor. Biochemical and Biophysical Research Communications. 2002;297:442–445. doi: 10.1016/s0006-291x(02)02009-0. [DOI] [PubMed] [Google Scholar]
- Copland JA, Ives KL, Simmons DJ, Soloff MS. Functional oxytocin receptors discovered in human osteoblasts. Endocrinology. 1999;140:4371–4374. doi: 10.1210/endo.140.9.7130. [DOI] [PubMed] [Google Scholar]
- Coss D, Yang L, Kuo CB, Xu X, Luben RA, Walker AM. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. American Journal of Physiology - Endocrinology and Metabolism. 2000;279:E1216–1225. doi: 10.1152/ajpendo.2000.279.6.E1216. [DOI] [PubMed] [Google Scholar]
- Crandall CJ, Tseng CH, Karlamangla AS, Finkelstein JS, Randolph JF, Jr, Thurston RC, Huang MH, Zheng H, Greendale GA. Serum sex steroid levels and longitudinal changes in bone density in relation to the final menstrual period. Journal of Clinical Endocrinology and Metabolism. 2013;98:E654–663. doi: 10.1210/jc.2012-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Zhao G, Liu R, Zheng M, Chen J, Wen J. FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR. J Lipid Res. 2012;53:909–917. doi: 10.1194/jlr.M025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus K, Wang X, Liu JL. A general IGF-I overexpression effectively rescued somatic growth and bone deficiency in mice caused by growth hormone receptor knockout. Growth Factors. 2009;27:438–447. doi: 10.3109/08977190903299270. [DOI] [PubMed] [Google Scholar]
- Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier AL, Petrosino S, Piscitelli F, Legros JJ, Geenen V, Foti M, et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One. 2011;6:e25565. doi: 10.1371/journal.pone.0025565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devleta B, Adem B, Senada S. Hypergonadotropic amenorrhea and bone density: new approach to an old problem. Journal of Bone and Mineral Metabolism. 2004;22:360–364. doi: 10.1007/s00774-004-0495-1. [DOI] [PubMed] [Google Scholar]
- Di Benedetto A, Sun L, Zambonin CG, Tamma R, Nico B, Calvano CD, Colaianni G, Ji Y, Mori G, Grano M, et al. Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16502–16507. doi: 10.1073/pnas.1419349111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Zhang Y, Li Q, Hu Y, Tao XJ, Liu BL, Ma JH, Li DM. Low Thyroid Stimulating Hormone Levels Are Associated with Low Bone Mineral Density in Femoral Neck in Elderly Women. Archives of Medical Research. 2016;47:310–314. doi: 10.1016/j.arcmed.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Drake MT, McCready LK, Hoey KA, Atkinson EJ, Khosla S. Effects of suppression of follicle-stimulating hormone secretion on bone resorption markers in postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 2010;95:5063–5068. doi: 10.1210/jc.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd C, Basillais A, Beaupied H, Breuil V, Wagner N, Scheideler M, Zaragosi LE, Massiera F, Lemichez E, Trajanoski Z, et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells. 2008;26:2399–2407. doi: 10.1634/stemcells.2008-0127. [DOI] [PubMed] [Google Scholar]
- Elabd SK, Sabry I, Hassan WB, Nour H, Zaky K. Possible neuroendocrine role for oxytocin in bone remodeling. Endocrine Regulations. 2007;41:131–141. [PubMed] [Google Scholar]
- Endo T, Kobayashi T. Expression of functional TSH receptor in white adipose tissues of hyt/hyt mice induces lipolysis in vivo. American Journal of Physiology - Endocrinology and Metabolism. 2012;302:E1569–1575. doi: 10.1152/ajpendo.00572.2011. [DOI] [PubMed] [Google Scholar]
- Feng Y, Zhu S, Antaris AL, Chen H, Xiao Y, Lu X, Jiang L, Diao S, Yu K, Wang Y, et al. Live imaging of follicle stimulating hormone receptors in gonads and bones using near infrared II fluorophore. Chem Sci. 2017;8:3703–3711. doi: 10.1039/c6sc04897h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. Journal of Clinical Endocrinology and Metabolism. 2010;95:186–193. doi: 10.1210/jc.2009-1625. [DOI] [PubMed] [Google Scholar]
- Fritton JC, Emerton KB, Sun H, Kawashima Y, Mejia W, Wu Y, Rosen CJ, Panus D, Bouxsein M, Majeska RJ, et al. Growth hormone protects against ovariectomy-induced bone loss in states of low circulating insulin-like growth factor (IGF-1) Journal of Bone and Mineral Research. 2010;25:235–246. doi: 10.1359/jbmr.090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CM, Moonga BS, Kovach JS. Cadmium, follicle-stimulating hormone, and effects on bone in women age 42–60 years, NHANES III. Environmental Research. 2010;110:105–111. doi: 10.1016/j.envres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Gankam Kengne F, Andres C, Sattar L, Melot C, Decaux G. Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM. 2008;101:583–588. doi: 10.1093/qjmed/hcn061. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin A, Reyes-Garcia R, Garcia-Castro JM, Rozas-Moreno P, Escobar-Jimenez F, Munoz-Torres M. Role of serum FSH measurement on bone resorption in postmenopausal women. Endocrine. 2012;41:302–308. doi: 10.1007/s12020-011-9541-7. [DOI] [PubMed] [Google Scholar]
- Geng W, Yan X, Du H, Cui J, Li L, Chen F. Immunization with FSHbeta fusion protein antigen prevents bone loss in a rat ovariectomy-induced osteoporosis model. Biochemical and Biophysical Research Communications. 2013;434:280–286. doi: 10.1016/j.bbrc.2013.02.116. [DOI] [PubMed] [Google Scholar]
- Gertz ER, Silverman NE, Wise KS, Hanson KB, Alekel DL, Stewart JW, Perry CD, Bhupathiraju SN, Kohut ML, Van Loan MD. Contribution of serum inflammatory markers to changes in bone mineral content and density in postmenopausal women: a 1-year investigation. Journal of Clinical Densitometry. 2010;13:277–282. doi: 10.1016/j.jocd.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay ML, Preisser JS, Hammett-Stabler CA, Renner JB, Rubin J. Follicle-stimulating hormone and bioavailable estradiol are less important than weight and race in determining bone density in younger postmenopausal women. Osteoporosis International. 2011;22:2699–2708. doi: 10.1007/s00198-010-1505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay ML, Specker BL, Li C, Hammett-Stabler CA, Renner JB, Rubin JE. Follicle-stimulating hormone is independently associated with lean mass but not BMD in younger postmenopausal women. Bone. 2012;50:311–316. doi: 10.1016/j.bone.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbly C, Martin B, Karpinski E, Harvey S. Growth hormone production and action in N1E-115 neuroblastoma cells. J Mol Neurosci. 2009;39:117–124. doi: 10.1007/s12031-009-9194-7. [DOI] [PubMed] [Google Scholar]
- Grimnes G, Emaus N, Joakimsen RM, Figenschau Y, Jorde R. The relationship between serum TSH and bone mineral density in men and postmenopausal women: the Tromso study. Thyroid. 2008;18:1147–1155. doi: 10.1089/thy.2008.0158. [DOI] [PubMed] [Google Scholar]
- Harbour DV, Kruger TE, Coppenhaver D, Smith EM, Meyer WJ., 3rd Differential expression and regulation of thyrotropin (TSH) in T cell lines. Molecular and Cellular Endocrinology. 1989;64:229–241. doi: 10.1016/0303-7207(89)90150-0. [DOI] [PubMed] [Google Scholar]
- Harvey S, Aramburo C. Growth Hormone: Not just a pituitary endocrine. J Endocr Disord. 2017;4:1024. [Google Scholar]
- Harvey S, Kakebeeke M, Sanders EJ. Growth hormone localization in the neural retina and retinal pigmented epithelium of embryonic chicks. J Mol Neurosci. 2004;22:139–145. doi: 10.1385/JMN:22:1-2:139. [DOI] [PubMed] [Google Scholar]
- Hase H, Ando T, Eldeiry L, Brebene A, Peng Y, Liu L, Amano H, Davies TF, Sun L, Zaidi M, et al. TNFalpha mediates the skeletal effects of thyroid-stimulating hormone. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12849–12854. doi: 10.1073/pnas.0600427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemstra KA, van der Deure WM, Peeters RP, Hamdy NA, Stokkel MP, Corssmit EP, Romijn JA, Visser TJ, Smit JW. Thyroid hormone independent associations between serum TSH levels and indicators of bone turnover in cured patients with differentiated thyroid carcinoma. European Journal of Endocrinology. 2008;159:69–76. doi: 10.1530/EJE-08-0038. [DOI] [PubMed] [Google Scholar]
- Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9:101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- Hoorn EJ, Rivadeneira F, van Meurs JB, Ziere G, Stricker BH, Hofman A, Pols HA, Zietse R, Uitterlinden AG, Zillikens MC. Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. Journal of Bone and Mineral Research. 2011;26:1822–1828. doi: 10.1002/jbmr.380. [DOI] [PubMed] [Google Scholar]
- Hori M. Tolvaptan for the treatment of hyponatremia and hypervolemia in patients with congestive heart failure. Future Cardiology. 2013;9:163–176. doi: 10.2217/fca.13.3. [DOI] [PubMed] [Google Scholar]
- Hull KL, Harvey S. Growth hormone and reproduction: a review of endocrine and autocrine/paracrine interactions. Int J Endocrinol. 2014;2014:234014. doi: 10.1155/2014/234014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Harbaugh CR. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiology & Behavior. 1989;45:1033–1041. doi: 10.1016/0031-9384(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14925–14930. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isales CM, Zaidi M, Blair HC. ACTH is a novel regulator of bone mass. Annals of the New York Academy of Sciences. 2010;1192:110–116. doi: 10.1111/j.1749-6632.2009.05231.x. [DOI] [PubMed] [Google Scholar]
- Karimifar M, Esmaili F, Salari A, Kachuei A, Faragzadegan Z, Karimifar M. Effects of Levothyroxine and thyroid stimulating hormone on bone loss in patients with primary hypothyroidism. Journal of Research in Pharmacy Practice. 2014;3:83–87. doi: 10.4103/2279-042X.141099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Furuhashi M, Suganuma N. Serum follicle-stimulating hormone level is a predictor of bone mineral density in patients with hormone replacement therapy. Archives of Gynecology and Obstetrics. 2004;269:192–195. doi: 10.1007/s00404-003-0532-7. [DOI] [PubMed] [Google Scholar]
- Kim MK, Yun KJ, Kim MH, Lim DJ, Kwon HS, Song KH, Kang MI, Baek KH. The effects of thyrotropin-suppressing therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Bone. 2015;71:101–105. doi: 10.1016/j.bone.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clinical Journal of the American Society of Nephrology. 2010;5:275–280. doi: 10.2215/CJN.06120809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JR, Wang HC. Characterization of a novel set of resident intrathyroidal bone marrow-derived hematopoietic cells: potential for immune-endocrine interactions in thyroid homeostasis. Journal of Experimental Biology. 2004;207:55–65. doi: 10.1242/jeb.00710. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Andersen O. The gonadotropin receptors FSH-R and LH-R of Atlantic halibut (Hippoglossus hippoglossus), 1: isolation of multiple transcripts encoding full-length and truncated variants of FSH-R. General and Comparative Endocrinology. 2008;156:584–594. doi: 10.1016/j.ygcen.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Krishnamra N, Seemoung J. Effects of acute and long-term administration of prolactin on bone 45Ca uptake, calcium deposit, and calcium resorption in weaned, young, and mature rats. Canadian Journal of Physiology and Pharmacology. 1996;74:1157–1165. [PubMed] [Google Scholar]
- Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Molecular Endocrinology. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RS, Ijiri S, Kight K, Swanson P, Dittman A, Alok D, Zohar Y, Trant JM. Cloning and functional expression of a thyrotropin receptor from the gonads of a vertebrate (bony fish): potential thyroid-independent role for thyrotropin in reproduction. Molecular and Cellular Endocrinology. 2000;167:1–9. doi: 10.1016/s0303-7207(00)00304-x. [DOI] [PubMed] [Google Scholar]
- Kumar RS, Ijiri S, Trant JM. Molecular biology of the channel catfish gonadotropin receptors: 2. Complementary DNA cloning, functional expression, and seasonal gene expression of the follicle-stimulating hormone receptor. Biology of Reproduction. 2001;65:710–717. doi: 10.1095/biolreprod65.3.710. [DOI] [PubMed] [Google Scholar]
- La Vignera S, Vicari E, Tumino S, Ciotta L, Condorelli R, Vicari LO, Calogero AE. L-thyroxin treatment and post-menopausal osteoporosis: relevance of the risk profile present in clinical history. Minerva Ginecologica. 2008;60:475–484. [PubMed] [Google Scholar]
- Lambrinoudaki I, Armeni E, Rizos D, Georgiopoulos G, Athanasouli F, Triantafyllou N, Panoulis K, Augoulea A, Creatsa M, Alexandrou A, et al. Indices of adiposity and thyroid hormones in euthyroid postmenopausal women. European Journal of Endocrinology. 2015;173:237–245. doi: 10.1530/EJE-15-0141. [DOI] [PubMed] [Google Scholar]
- Laron Z. Natural history of the classical form of primary growth hormone (GH) resistance (Laron syndrome) Journal of Pediatric Endocrinology & Metabolism 12 Suppl. 1999;1:231–249. [PubMed] [Google Scholar]
- Laron Z, Klinger B, Silbergeld A. Patients with Laron syndrome have Osteopenia/Osteoporosis. Journal of Bone and Mineral Research. 1999;14:156–157. doi: 10.1359/jbmr.1999.14.1.156. [DOI] [PubMed] [Google Scholar]
- Lawson EA, Ackerman KE, Estella NM, Guereca G, Pierce L, Sluss PM, Bouxsein ML, Klibanski A, Misra M. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. European Journal of Endocrinology. 2013;168:457–464. doi: 10.1530/EJE-12-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Kim KM, Lee EY, Song MK, Kang DR, Kim HC, Youm Y, Yun YM, Park HY, Kim CO, et al. Low Normal TSH levels are Associated with Impaired BMD and Hip Geometry in the Elderly. Aging and Disease. 2016;7:734–743. doi: 10.14336/AD.2016.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Jiang Y, Sun J, Liang J, Jin Y. MR spectroscopy for assessing the effects of oxytocin on marrow adipogenesis induced by glucocorticoid in rabbits. Acta Radiol. 2016;57:701–707. doi: 10.1177/0284185115599804. [DOI] [PubMed] [Google Scholar]
- Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, Abu-Amer W, Izadmehr S, Zhou B, Shin AC, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546:107–112. doi: 10.1038/nature22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RD, Chen RX, Li WR, Huang YL, Li WH, Cai GR, Zhang H. The Glu727 Allele of Thyroid Stimulating Hormone Receptor Gene is Associated with Osteoporosis. North American Journal of Medical Sciences. 2012;4:300–304. doi: 10.4103/1947-2714.98588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Cheng Y, Fan M, Chen D, Bian Z. FSH aggravates periodontitis-related bone loss in ovariectomized rats. Journal of Dental Research. 2010a;89:366–371. doi: 10.1177/0022034509358822. [DOI] [PubMed] [Google Scholar]
- Liu S, Cheng Y, Xu W, Bian Z. Protective effects of follicle-stimulating hormone inhibitor on alveolar bone loss resulting from experimental periapical lesions in ovariectomized rats. Journal of Endodontics. 2010b;36:658–663. doi: 10.1016/j.joen.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Liu X, Shimono K, Zhu LL, Li J, Peng Y, Imam A, Iqbal J, Moonga S, Colaianni G, Su C, et al. Oxytocin deficiency impairs maternal skeletal remodeling. Biochemical and Biophysical Research Communications. 2009;388:161–166. doi: 10.1016/j.bbrc.2009.07.148. [DOI] [PubMed] [Google Scholar]
- Liu XM, Chan HC, Ding GL, Cai J, Song Y, Wang TT, Zhang D, Chen H, Yu MK, Wu YT, et al. FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway. Aging Cell. 2015;14:409–420. doi: 10.1111/acel.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotinun S, Limlomwongse L, Krishnamra N. The study of a physiological significance of prolactin in the regulation of calcium metabolism during pregnancy and lactation in rats. Canadian Journal of Physiology and Pharmacology. 1998;76:218–228. [PubMed] [Google Scholar]
- Lotinun S, Limlomwongse L, Sirikulchayanonta V, Krishnamra N. Bone calcium turnover, formation, and resorption in bromocriptine- and prolactin-treated lactating rats. Endocrine. 2003;20:163–170. doi: 10.1385/ENDO:20:1-2:163. [DOI] [PubMed] [Google Scholar]
- Lu S, Guan Q, Liu Y, Wang H, Xu W, Li X, Fu Y, Gao L, Zhao J, Wang X. Role of extrathyroidal TSHR expression in adipocyte differentiation and its association with obesity. Lipids Health Dis. 2012;11:17. doi: 10.1186/1476-511X-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukefahr AL, Frye JB, Wright LE, Marion SL, Hoyer PB, Funk JL. Decreased bone mineral density in rats rendered follicle-deplete by an ovotoxic chemical correlates with changes in follicle-stimulating hormone and inhibin A. Calcified Tissue International. 2012;90:239–249. doi: 10.1007/s00223-011-9565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Latif R, Davies TF. Thyrotropin-independent induction of thyroid endoderm from embryonic stem cells by activin A. Endocrinology. 2009;150:1970–1975. doi: 10.1210/en.2008-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Morshed S, Latif R, Zaidi M, Davies TF. The influence of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on osteoclastogenesis. Thyroid. 2011;21:897–906. doi: 10.1089/thy.2010.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y, Aoyama M, Sakamoto K, Jojima T, Aso Y, Takasu K, Takenosihita S, Shimomura K. Impact of sex, fat distribution and initial body weight on oxytocin's body weight regulation. Sci Rep. 2017;7:8599. doi: 10.1038/s41598-017-09318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY) 2011;3:1169–1177. doi: 10.18632/aging.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144:2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- Martin BT, List EO, Kopchick JJ, Sauve Y, Harvey S. Selective inner retinal dysfunction in growth hormone transgenic mice. Growth Horm IGF Res. 2011;21:219–227. doi: 10.1016/j.ghir.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Moreno CG, Fleming T, Carranza M, Avila-Mendoza J, Luna M, Harvey S, Aramburo C. Growth hormone protects against kainate excitotoxicity and induces BDNF and NT3 expression in chicken neuroretinal cells. Exp Eye Res. 2018;166:1–12. doi: 10.1016/j.exer.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Martini G, Gennari L, De Paola V, Pilli T, Salvadori S, Merlotti D, Valleggi F, Campagna S, Franci B, Avanzati A, et al. The effects of recombinant TSH on bone turnover markers and serum osteoprotegerin and RANKL levels. Thyroid. 2008;18:455–460. doi: 10.1089/thy.2007.0166. [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Porcelli T, Patelli I, Vescovi PP, Giustina A. Serum TSH values and risk of vertebral fractures in euthyroid post-menopausal women with low bone mineral density. Bone. 2010;46:747–751. doi: 10.1016/j.bone.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Sorvillo F, Piscopo M, Cioffi M, Pilla P, Biondi B, Iorio S, Giustina A, Amato G, Carella C. Recombinant human TSH modulates in vivo C-telopeptides of type-1 collagen and bone alkaline phosphatase, but not osteoprotegerin production in postmenopausal women monitored for differentiated thyroid carcinoma. Journal of Bone and Mineral Research. 2005;20:480–486. doi: 10.1359/JBMR.041126. [DOI] [PubMed] [Google Scholar]
- Meaney AM, Smith S, Howes OD, O'Brien M, Murray RM, O'Keane V. Effects of long-term prolactin-raising antipsychotic medication on bone mineral density in patients with schizophrenia. British Journal of Psychiatry. 2004;184:503–508. doi: 10.1192/bjp.184.6.503. [DOI] [PubMed] [Google Scholar]
- Meher BR, Dixit A, Bousfield GR, Lushington GH. Glycosylation Effects on FSH-FSHR Interaction Dynamics: A Case Study of Different FSH Glycoforms by Molecular Dynamics Simulations. PLoS One. 2015;10:e0137897. doi: 10.1371/journal.pone.0137897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menagh PJ, Turner RT, Jump DB, Wong CP, Lowry MB, Yakar S, Rosen CJ, Iwaniec UT. Growth hormone regulates the balance between bone formation and bone marrow adiposity. Journal of Bone and Mineral Research. 2010;25:757–768. doi: 10.1359/jbmr.091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza N, Quereda F, Presa J, Salamanca A, Sanchez-Borrego R, Vazquez F, Martinez Astorquiza T. Estrogen-related genes and postmenopausal osteoporosis risk. Climacteric. 2012;15:587–593. doi: 10.3109/13697137.2012.656160. [DOI] [PubMed] [Google Scholar]
- Mikosch P, Kerschan-Schindl K, Woloszczuk W, Stettner H, Kudlacek S, Kresnik E, Gallowitsch HJ, Lind P, Pietschmann P. High cathepsin K levels in men with differentiated thyroid cancer on suppressive L-thyroxine therapy. Thyroid. 2008;18:27–33. doi: 10.1089/thy.2007.0186. [DOI] [PubMed] [Google Scholar]
- Morris MS. The association between serum thyroid-stimulating hormone in its reference range and bone status in postmenopausal American women. Bone. 2007;40:1128–1134. doi: 10.1016/j.bone.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. American Journal of Physiology - Endocrinology and Metabolism. 2012;302:E134–144. doi: 10.1152/ajpendo.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscogiuri G, Sorice GP, Mezza T, Prioletta A, Lassandro AP, Pirronti T, Della Casa S, Pontecorvi A, Giaccari A. High-normal TSH values in obesity: is it insulin resistance or adipose tissue's guilt? Obesity (Silver Spring) 2013;21:101–106. doi: 10.1002/oby.20240. [DOI] [PubMed] [Google Scholar]
- Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. Journal of Bone and Mineral Research. 2000;15:129–137. doi: 10.1359/jbmr.2000.15.1.129. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh HM, Park YS, Lee J, Lee W. A cross-sectional study to examine the correlation between serum TSH levels and the osteoporosis of the lumbar spine in healthy women with normal thyroid function. Osteoporosis International. 2015;26:997–1003. doi: 10.1007/s00198-014-2906-z. [DOI] [PubMed] [Google Scholar]
- Novack DV. TSH, the bone suppressing hormone. Cell. 2003;115:129–130. doi: 10.1016/s0092-8674(03)00812-2. [DOI] [PubMed] [Google Scholar]
- Pallinger E, Csaba G. A hormone map of human immune cells showing the presence of adrenocorticotropic hormone, triiodothyronine and endorphin in immunophenotyped white blood cells. Immunology. 2008;123:584–589. doi: 10.1111/j.1365-2567.2007.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podfigurna-Stopa A, Pludowski P, Jaworski M, Lorenc R, Genazzani AR, Meczekalski B. Skeletal status and body composition in young women with functional hypothalamic amenorrhea. Gynecological Endocrinology. 2012;28:299–304. doi: 10.3109/09513590.2011.613972. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Yao J, Wu X, Zhou B, Shao H, Hua T, Xiong Z, Tang G. Longitudinal assessment of oxytocin efficacy on bone and bone marrow fat masses in a rabbit osteoporosis model through 3.0-T magnetic resonance spectroscopy and micro-CT. Osteoporosis International. 2015;26:1081–1092. doi: 10.1007/s00198-014-2933-9. [DOI] [PubMed] [Google Scholar]
- Randolph JF, Jr, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. Journal of Clinical Endocrinology and Metabolism. 2003;88:1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- Rendina D, Gianfrancesco F, De Filippo G, Merlotti D, Esposito T, Mingione A, Nuti R, Strazzullo P, Mossetti G, Gennari L. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. European Journal of Endocrinology. 2010;163:165–172. doi: 10.1530/EJE-10-0043. [DOI] [PubMed] [Google Scholar]
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. American Journal of Medicine. 2006;119:e71–78. doi: 10.1016/j.amjmed.2005.09.026. 71. [DOI] [PubMed] [Google Scholar]
- Ritter V, Thuering B, Saint Mezard P, Luong-Nguyen NH, Seltenmeyer Y, Junker U, Fournier B, Susa M, Morvan F. Follicle-stimulating hormone does not impact male bone mass in vivo or human male osteoclasts in vitro. Calcified Tissue International. 2008;82:383–391. doi: 10.1007/s00223-008-9134-5. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Tourkova I, Wang Y, Sharrow AC, Landau MS, Yaroslavskiy BB, Sun L, Zaidi M, Blair HC. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochemical and Biophysical Research Communications. 2010;394:12–17. doi: 10.1016/j.bbrc.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath TK, Simic P, Sendak R, Draca N, Bowe AE, O'Brien S, Schiavi SC, McPherson JM, Vukicevic S. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. Journal of Bone and Mineral Research. 2007;22:849–859. doi: 10.1359/jbmr.070302. [DOI] [PubMed] [Google Scholar]
- Sandhu HS, Gilles E, DeVita MV, Panagopoulos G, Michelis MF. Hyponatremia associated with large-bone fracture in elderly patients. International Urology and Nephrology. 2009;41:733–737. doi: 10.1007/s11255-009-9585-2. [DOI] [PubMed] [Google Scholar]
- Schaefer JS, Klein JR. A novel thyroid stimulating hormone beta-subunit isoform in human pituitary, peripheral blood leukocytes, and thyroid. Gen Comp Endocrinol. 2009;162:241–244. doi: 10.1016/j.ygcen.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr M, Marengi DA, Pulumo RL, Yu E, Eddy KT, Klibanski A, Miller KK, Lawson EA. Oxytocin and Its Relationship to Body Composition, Bone Mineral Density, and Hip Geometry Across the Weight Spectrum. Journal of Clinical Endocrinology and Metabolism. 2017;102:2814–2824. doi: 10.1210/jc.2016-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Rinaman L, Vollmer RR, Amico JA. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2007;292:R1828–1833. doi: 10.1152/ajpregu.00826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert-Klauss V, Fillenberg S, Schneider H, Luppa P, Mueller D, Kiechle M. Bone loss in premenopausal, perimenopausal and postmenopausal women: results of a prospective observational study over 9 years. Climacteric. 2012;15:433–440. doi: 10.3109/13697137.2012.658110. [DOI] [PubMed] [Google Scholar]
- Sejling AS, Pedersen-Bjergaard U, Eiken P. Syndrome of inappropriate ADH secretion and severe osteoporosis. Journal of Clinical Endocrinology and Metabolism. 2012;97:4306–4310. doi: 10.1210/jc.2012-2031. [DOI] [PubMed] [Google Scholar]
- Seriwatanachai D, Charoenphandhu N, Suthiphongchai T, Krishnamra N. Prolactin decreases the expression ratio of receptor activator of nuclear factor kappaB ligand/osteoprotegerin in human fetal osteoblast cells. Cell Biology International. 2008a;32:1126–1135. doi: 10.1016/j.cellbi.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Seriwatanachai D, Thongchote K, Charoenphandhu N, Pandaranandaka J, Tudpor K, Teerapornpuntakit J, Suthiphongchai T, Krishnamra N. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone. 2008b;42:535–546. doi: 10.1016/j.bone.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Smith EM, Phan M, Kruger TE, Coppenhaver DH, Blalock JE. Human lymphocyte production of immunoreactive thyrotropin. Proc Natl Acad Sci U S A. 1983a;80:6010–6013. doi: 10.1073/pnas.80.19.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Phan M, Kruger TE, Coppenhaver DH, Blalock JE. Human lymphocyte production of immunoreactive thyrotropin. Proceedings of the National Academy of Sciences of the United States of America. 1983b;80:6010–6013. doi: 10.1073/pnas.80.19.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers M, Eyre D, Hollis BW, Randolph JF, Shapiro B, Jannausch ML, Crutchfield M. Biochemical markers of bone turnover in lactating and nonlactating postpartum women. Journal of Clinical Endocrinology and Metabolism. 1995;80:2210–2216. doi: 10.1210/jcem.80.7.7608281. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM, Ettinger B. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporosis International. 2003;14:191–197. doi: 10.1007/s00198-002-1329-4. [DOI] [PubMed] [Google Scholar]
- Su XY, Zou X, Chen QZ, Zeng YH, Shao Y, He BC, Liu H. Follicle-Stimulating Hormone beta-Subunit Potentiates Bone Morphogenetic Protein 9-Induced Osteogenic Differentiation in Mouse Embryonic Fibroblasts. Journal of Cellular Biochemistry. 2017;118:1792–1802. doi: 10.1002/jcb.25849. [DOI] [PubMed] [Google Scholar]
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- Sun L, Tamma R, Yuen T, Colaianni G, Ji Y, Cuscito C, Bailey J, Dhawan S, Lu P, Calvano CD, et al. Functions of vasopressin and oxytocin in bone mass regulation. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:164–169. doi: 10.1073/pnas.1523762113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Vukicevic S, Baliram R, Yang G, Sendak R, McPherson J, Zhu LL, Iqbal J, Latif R, Natrajan A, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4289–4294. doi: 10.1073/pnas.0712395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Zhang Z, Zhu LL, Peng Y, Liu X, Li J, Agrawal M, Robinson LJ, Iqbal J, Blair HC, et al. Further evidence for direct pro-resorptive actions of FSH. Biochemical and Biophysical Research Communications. 2010;394:6–11. doi: 10.1016/j.bbrc.2010.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Zhu LL, Lu P, Yuen T, Li J, Ma R, Baliram R, Moonga SS, Liu P, Zallone A, et al. Genetic confirmation for a central role for TNFalpha in the direct action of thyroid stimulating hormone on the skeleton. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9891–9896. doi: 10.1073/pnas.1308336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svare A, Nilsen TI, Bjoro T, Forsmo S, Schei B, Langhammer A. Hyperthyroid levels of TSH correlate with low bone mineral density: the HUNT 2 study. European Journal of Endocrinology. 2009;161:779–786. doi: 10.1530/EJE-09-0139. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. Journal of Clinical Endocrinology and Metabolism. 1995;80:573–579. doi: 10.1210/jcem.80.2.7852523. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- Tamma R, Colaianni G, Zhu LL, Di Benedetto A, Greco G, Montemurro G, Patano N, Strippoli M, Vergari R, Mancini L, et al. Oxytocin is an anabolic bone hormone. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7149–7154. doi: 10.1073/pnas.0901890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma R, Sun L, Cuscito C, Lu P, Corcelli M, Li J, Colaianni G, Moonga SS, Di Benedetto A, Grano M, et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18644–18649. doi: 10.1073/pnas.1318257110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston RC, Sowers MR, Sternfeld B, Gold EB, Bromberger J, Chang Y, Joffe H, Crandall CJ, Waetjen LE, Matthews KA. Gains in body fat and vasomotor symptom reporting over the menopausal transition: the study of women's health across the nation. Am J Epidemiol. 2009;170:766–774. doi: 10.1093/aje/kwp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. Journal of Neuroscience. 2010;30:3803–3812. doi: 10.1523/JNEUROSCI.5444-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourkova IL, Witt MR, Li L, Larrouture Q, Liu L, Luo J, Robinson LJ, Blair HC. Follicle stimulating hormone receptor in mesenchymal stem cells integrates effects of glycoprotein reproductive hormones. Annals of the New York Academy of Sciences. 2015;1335:100–109. doi: 10.1111/nyas.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Deure WM, Uitterlinden AG, Hofman A, Rivadeneira F, Pols HA, Peeters RP, Visser TJ. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: the Rotterdam Study. Clinical Endocrinology. 2008;68:175–181. doi: 10.1111/j.1365-2265.2007.03016.x. [DOI] [PubMed] [Google Scholar]
- van Rijn LE, Pop VJ, Williams GR. Low bone mineral density is related to high physiological levels of free thyroxine in peri-menopausal women. European Journal of Endocrinology. 2014;170:461–468. doi: 10.1530/EJE-13-0769. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Barsony J, Sugimura Y, Tian Y, Adams DJ, Carter EA, Resnick HE. Hyponatremia-induced osteoporosis. Journal of Bone and Mineral Research. 2010;25:554–563. doi: 10.1359/jbmr.090827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibede N, Hauser F, Williamson M, Grimmelikhuijzen CJ. Genomic organization of a receptor from sea anemones, structurally and evolutionarily related to glycoprotein hormone receptors from mammals. Biochemical and Biophysical Research Communications. 1998;252:497–501. doi: 10.1006/bbrc.1998.9661. [DOI] [PubMed] [Google Scholar]
- Vincent BH, Montufar-Solis D, Teng BB, Amendt BA, Schaefer J, Klein JR. Bone marrow cells produce a novel TSHbeta splice variant that is upregulated in the thyroid following systemic virus infection. Genes & Immunity. 2009a;10:18–26. doi: 10.1038/gene.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent BH, Montufar-Solis D, Teng BB, Amendt BA, Schaefer J, Klein JR. Bone marrow cells produce a novel TSHbeta splice variant that is upregulated in the thyroid following systemic virus infection. Genes Immun. 2009b;10:18–26. doi: 10.1038/gene.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Song Y, Chen Y, Wang ES, Zheng D, Qu F, Zhou JH. Correlation analysis for follicle-stimulating hormone and C-terminal cross-linked telopetides of type i collagen in menopausal transition women with osteoporosis. International Journal of Clinical and Experimental Medicine. 2015a;8:2417–2422. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang W, Yu C, Zhang X, Zhang H, Guan Q, Zhao J, Xu J. Follicle-Stimulating Hormone Increases the Risk of Postmenopausal Osteoporosis by Stimulating Osteoclast Differentiation. PLoS One. 2015b;10:e0134986. doi: 10.1371/journal.pone.0134986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Smith AW, Palmer FL, Tuttle RM, Mahrous A, Nixon IJ, Patel SG, Ganly I, Fagin JA, Boucai L. Thyrotropin suppression increases the risk of osteoporosis without decreasing recurrence in ATA low- and intermediate-risk patients with differentiated thyroid carcinoma. Thyroid. 2015c;25:300–307. doi: 10.1089/thy.2014.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Lan L, Li T, Li J, Li Y. The effect of oxytocin on osseointegration of titanium implant in ovariectomized rats. Connective Tissue Research. 2016;57:220–225. doi: 10.3109/03008207.2016.1141902. [DOI] [PubMed] [Google Scholar]
- Waring AC, Harrison S, Fink HA, Samuels MH, Cawthon PM, Zmuda JM, Orwoll ES, Bauer DC, Osteoporotic Fractures in Men S A prospective study of thyroid function, bone loss, and fractures in older men: The MrOS study. Journal of Bone and Mineral Research. 2013;28:472–479. doi: 10.1002/jbmr.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman RP, Tepper PG, Crawford S, Finkelstein JS, Sutton-Tyrrell K, Thurston RC, Santoro N, Sternfeld B, Greendale GA. Do changes in sex steroid hormones precede or follow increases in body weight during the menopause transition? Results from the Study of Women's Health Across the Nation. Journal of Clinical Endocrinology and Metabolism. 2012;97:E1695–1704. doi: 10.1210/jc.2012-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte T, Volzke H, Lerch MM, Hegenscheid K, Friedrich N, Ittermann T, Batsis JA. Association between Serum Thyroid-Stimulating Hormone Levels and Visceral Adipose Tissue: A Population-Based Study in Northeast Germany. Eur Thyroid J. 2017;6:12–19. doi: 10.1159/000450977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XY, Wu XP, Xie H, Zhang H, Peng YQ, Yuan LQ, Su X, Luo XH, Liao EY. Age-related changes in biochemical markers of bone turnover and gonadotropin levels and their relationship among Chinese adult women. Osteoporosis International. 2010;21:275–285. doi: 10.1007/s00198-009-0943-9. [DOI] [PubMed] [Google Scholar]
- Wu Y, Torchia J, Yao W, Lane NE, Lanier LL, Nakamura MC, Humphrey MB. Bone microenvironment specific roles of ITAM adapter signaling during bone remodeling induced by acute estrogen-deficiency. PLoS One. 2007;2:e586. doi: 10.1371/journal.pone.0000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski JJ. The evolutionary origins of maternal calcium and bone metabolism during lactation. Journal of Mammary Gland Biology and Neoplasia. 2002;7:267–276. doi: 10.1023/a:1022800716196. [DOI] [PubMed] [Google Scholar]
- Xu ZR, Wang AH, Wu XP, Zhang H, Sheng ZF, Wu XY, Xie H, Luo XH, Liao EY. Relationship of age-related concentrations of serum FSH and LH with bone mineral density, prevalence of osteoporosis in native Chinese women. Clinica Chimica Acta. 2009;400:8–13. doi: 10.1016/j.cca.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, et al. Circulating levels of IGF-1 directly regulate bone growth and density. Journal of Clinical Investigation. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamoah K, Brebene A, Baliram R, Inagaki K, Dolios G, Arabi A, Majeed R, Amano H, Wang R, Yanagisawa R, et al. High-mobility group box proteins modulate tumor necrosis factor-alpha expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Molecular Endocrinology. 2008;22:1141–1153. doi: 10.1210/me.2007-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, 3rd, Shepard E, DeVries AC, Zimmer A, LaMarca ME, Ginns EI, Amico J, Nelson RJ, Hennighausen L, Wagner KU. Targeted reduction of oxytocin expression provides insights into its physiological roles. Advances in Experimental Medicine and Biology. 1998;449:231–240. doi: 10.1007/978-1-4615-4871-3_30. [DOI] [PubMed] [Google Scholar]
- Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Sun L, Davies TF, Abe E. Low TSH triggers bone loss: fact or fiction? Thyroid. 2006;16:1075–1076. doi: 10.1089/thy.2006.16.1075. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, Zhu LL, Liu X, Li J, Peng Y, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallone A. Direct and indirect estrogen actions on osteoblasts and osteoclasts. Annals of the New York Academy of Sciences. 2006;1068:173–179. doi: 10.1196/annals.1346.019. [DOI] [PubMed] [Google Scholar]
- Zhang G, Cai D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. American Journal of Physiology - Endocrinology and Metabolism. 2011;301:E1004–1012. doi: 10.1152/ajpendo.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Sun H, Chen L, Zheng J, Hu X, Wang S, Chen T. Relationship between serum TSH level with obesity and NAFLD in euthyroid subjects. Journal of Huazhong University of Science and Technology - Medical sciences. 2012;32:47–52. doi: 10.1007/s11596-012-0008-8. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, Tourkova IL, Li J, Davies TF, Sun L, et al. Blocking antibody to the beta-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2012a;109:14574–14579. doi: 10.1073/pnas.1212806109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Tourkova I, Yuen T, Robinson LJ, Bian Z, Zaidi M, Blair HC. Blocking FSH action attenuates osteoclastogenesis. Biochemical and Biophysical Research Communications. 2012b;422:54–58. doi: 10.1016/j.bbrc.2012.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zofkova I, Hill M. Biochemical markers of bone remodeling correlate negatively with circulating TSH in postmenopausal women. Endocrine Regulations. 2008;42:121–127. [PubMed] [Google Scholar]