Scheme 2.
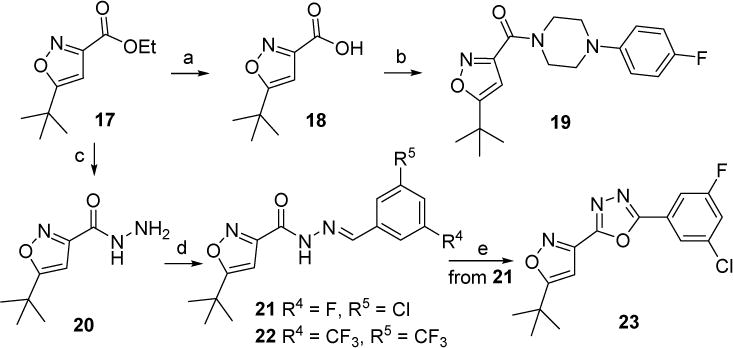
Synthetic route of new compounds 19, 22 and 23. Reagents and conditions: (a) LiOH·H2O, CH3OH/H2O, rt., 95%; (b) 1-(4-fluorophenyl) piperazine, HBTU, DIPEA, DCM, 81%; (c) NH2NH2, EtOH, reflux, used directly for the next step; (d) 3-chloro-5-fluorobenzaldehyde (for 21) or 3,5-bis(trifluoromethyl)benzaldehyde (for 22), EtOH, rt. 44% for 21 (two steps). 59% for 22 (two steps); (e) I2, K2CO3, DMSO, 110 °C, 13%.
