Scheme 3.
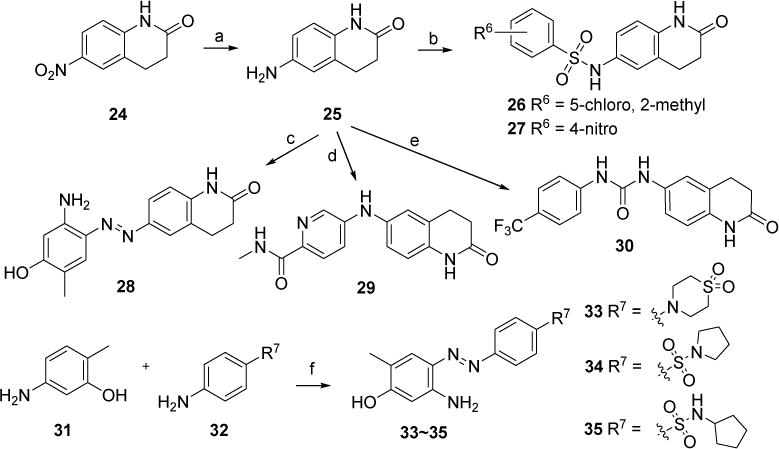
Synthetic route of new compounds 26~30 and 33~35. Reagents and conditions: (a) Zn, NH4Cl, MeOH/H2O, 80 °C, quant. (b) 5-chloro-2-methoxybenzenesulfonyl chloride (for 26) or 4-nitrobenzenesulfonyl chloride (for 27), Et3N, DMF, rt., 29% for 26, 13% for 27; (c) 5-amino-2-methylphenol, tert-butyl nitrite, 38% HCl (aq.), K2CO3, MeOH/CH3CN/H2O, 0°C, 81%. (d) 5-bromo-N-methylpicolinamide, Pd(OAc)2, xantphos, Cs2CO3, 1,4-dioxane, 110 °C, 63%. (e) 1-isocyanato-4-(trifluoromethyl)benzene, DCM, rt., 41%; (f) tert-butyl nitrite, 38% HCl (aq.), K2CO3, MeOH/CH3CN/H2O, 0 °C, 67% for 33, 92% for 34, 81% for 35.
