Abstract
Robust physiological circadian rhythms form an integral part of wellbeing. The aging process has been found to negatively impact systems that drive circadian physiology, typically manifesting as symptoms associated with abnormal/ disrupted sleeping patterns. Here, we investigated the age-related decline in light-driven circadian entrainment in male C57BL/6J mice. We compared light-driven resetting of circadian behavioural activity in young (1-2 months) and old (14-18 months) mice and explored alterations in the glutamatergic pathway at the level of the circadian pacemaker, the suprachiasmatic nucleus (SCN). Aged animals showed a significant reduction in sensitivity to behavioural phase-resetting by light. We show that this change was through alterations in NMDA signalling at the SCN, where NMDA, a glutamatergic agonist, was less potent in inducing clock resetting. Finally, we show that this shift in NMDA sensitivity was through the reduced SCN expression of this receptor's NR2B subunit. Only in young animals did an NR2B antagonist attenuate behavioural resetting. These results can help target treatments that aim to improve both physiological and behavioural circadian entrainment in aged populations.
Keywords: Aging, Circadian rhythm, NMDA, NR2B, Glutamate
1. Introduction
Aging is commonly associated with a loss in expression of circadian (daily) rhythms, which can lead to problems in sleep, cognition, and social function. To assist in prevention or amelioration of these age-related losses, we need to better understand the underlying causes. The circadian system in mammals is well characterized. The central pacemaker, the suprachiasmatic nucleus (SCN) of the hypothalamus, generates multiple rhythmic outputs that entrain circadian clocks in tissues throughout the body (Morin and Allen, 2006; Reppert and Weaver, 2002). Cells in the SCN are entrained to the environmental light-dark cycle via a specialized input pathway from the retina (Lucas et al., 2012). Aging has the potential of influencing all aspects of the circadian entraining circuitry, from the eye through to SCN input and ultimately output signalling.
The eye is known to undergo structural changes with age which can also impact function (Lin et al., 2016). Light information is decoded by retinal photoreceptors of different types: rods, cones and a subset of retinal ganglion cells expressing the melanopsin photopigment (Lucas, 2003). These cells provide visual cues of our surroundings as well as encode the irradiance of varying levels of ambient light. It is this latter physiological attribute that dictates many of the non-visual light driven processes, such as circadian entrainment, pupil size, pineal melatonin production and sleep propensity (Lucas et al., 2012). With healthy aging both visual acuity and color contrast show gradual deterioration over time. However, progressive, and often relatively rapid, declines in the visual processing are often due to age-associated health disorders such as macular degeneration and glaucoma, or more centrally through various forms of dementia (Maynard et al., 2017; Wulff, 2015).
Changes in the SCN with age have already been reported in laboratory animals such as mice, rats, and hamsters. These include altered expression of primary neuropeptides typically responsible for SCN cell orchestration, decreases in glucose uptake and declines in oscillatory activity of SCN neurons (Biello, 2009; Duncan et al., 2001; Kawakami et al., 1997; Krajnak et al., 1998; Madeira et al., 1995; Satinoff et al., 1993; Wise et al., 1988). SCN output function appears to be compromised with age, given that multiple studies report dampened amplitude of the circadian rhythm in neuronal spontaneous cell firing (Farajnia et al., 2012; Leise et al., 2013; Nakamura et al., 2011). We have previously shown that voluntary exercise, a non-photic stimulus, appears to remain an effective cue in aged mice. We have demonstrated that SCN firing rate, circadian gene PER2∷LUC expression, and phase resetting to light can all respond to non-photic input in aged mice (Leise et al., 2013). In addition, a lack of exercise can negatively impact locomotor scale invariance in aged mice, providing further evidence for the importance of behavioural intervention in healthy aging (Gu et al., 2015). On the other hand, it is unclear if photic responses of SCN cells are impaired with age.
Light-driven SCN responses are mediated by the excitatory neurotransmitter glutamate. Shifts in SCN cell firing rhythms on application of glutamate, in vitro, mimic those of light in vivo (Ebling, 1996). In addition, the action of glutamate, in part, has been attributed to the N-Methyl-D-aspartate (NMDA) receptor, where light-induced behavioural shifts were blocked by an NMDA receptor antagonist in vivo (Colwell et al., 1990). Further, direct application of the glutamatergic agonist NMDA to the SCN results in light-like behavioural resetting (Ding et al., 1994).
The NMDA receptor is a ligand-gated ion channel that consists of four membrane-bound subunits, two NR1 and one or more NR2 (NR2A, NR2B, NR2C, NR2D, NR3A, NR3B) subunits (Cull-Candy et al., 2001; Flores-Soto et al., 2012; Kumar, 2015; McBain and Mayer, 1994). The NR2 subunit defines the pharmacological characteristics of the receptor. The NR2B subunit has been shown to be important in the mediation of light signals to the circadian clock during the early and late night (Wang et al., 2008). Inhibition of NR2B-expressing receptors by the selective antagonist ifenprodil attenuated both phase delays and advances to light. Overall declines in the expression of NMDA receptors have been shown in a range of aged mammals from humans to rodents (Magnusson, 2000; Piggott et al., 1992). In particular, a decrease in NR2B expression has been found in most cortical regions and dentate granule cells of aged mice (Magnusson, 2000).
Here we investigate the impact of light sensitivity and NMDA signalling on circadian resetting in aged mice. Our studies investigate the impact at both the level of the eye and the SCN. We hypothesize circadian deficits in senescence are the result of changes to the photic input pathway driven by alterations in NMDA signalling within the SCN.
2. Methods
2.1. Animals
Male C57BL/6J mice (purchased from Charles River (Kent, UK, [SMB, GSL]) or Jackson Laboratories (MA, USA, [MEH] and bred in-house) were housed in groups of 1-4 prior to experimentation and then individually housed, with food and water provided ad libitum. Young animals were defined as mice aged between 1-2 months; aged mice were defined as animals aged between 14-18 months. Environmental room lighting consisted of white fluorescent strips providing approximately 250-350 lux at the level of the cage. All mice were maintained under a 12:12 h LD cycle for a minimum of 14 days prior to being assigned to an experimental group. All experimental procedures were performed with approval from the Universities of Glasgow [SMB] and Kent [GSL] Ethical Review Committees (UK) and in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986. All experimental work with animals at Smith College (USA [MEH]) was approved by the Institutional Animal Care and Use Committee and the work was conducted in an AAALAC-accredited animal facility.
2.2. Surgery
Mice (University of Glasgow, UK) were anaesthetised with a ketamine-xylazine mix (50mg/Kg, 20mg/Kg respectively) administered intraperitoneally (i.p) on the day of surgery. Guide cannulas were stereotaxically aimed at the SCN (coordinates relative to bregma: AP -0.4 mm, lateral 0 mm, DV -4.7 mm) and fixed to the skull with mounting screws and dental cement. Mice were left to recover for 14 days under a 12:12 LD cycle before being placed in cages with running wheels.
Cannula placements were identified by histological and/or functional assessment. Functional cannula placements were determined by an initial microinjection of NPY (Calbiochem, UK) at CT 6 (200nl; 1ng/nl). Mice who showed functional evidence of placement by shifts of at least 110 minutes were included in further cannula tests.
Histology: animals were killed using an overdose of sodium pentobarbital (approximately 160 mg/kg, i.p.) and given an intracerebral injection of India ink (200 nl) with the same length of injector used in the experiment. They were then perfused transcardially with 0.9% saline and 10% formaldehyde. The brains were postfixed in 10% formalin followed by 30% sucrose. Frontal sections (40 μm) were cut using a cryostat. Slices containing the SCN were mounted on Polysine microslides and stained for Nissl substance with Cresyl Violet. The distance from the injection site to the margin of the SCN was measured along the anterior–posterior, dorsal–ventral and lateral axes. The greatest of the three measurements was taken as the distance from the SCN. No data from animals with placements more than 400 μm from the margin of the SCN was included in analyses.
2.3. Circadian photoentrainment
Phase shifting experiments (Universities of Kent and Glasgow, UK) were performed using an Aschoff type I protocol. Animals were individually housed and placed under constant conditions of darkness (21 ± 2°C) 10 days prior to experimental treatment. Cages were fitted with running wheels and wheel revolutions were recorded in 1-min bins and analysed using ClockLab (Actimetrics, IL, USA). Under dim red light, groups of young (n= 14) and aged (n= 14) mice were removed from their cages and exposed to a 15 minute white fluorescent light (30 lux or 300 lux) at CT 14 (2 hours after activity onset).
Phase shifts to the glutamatergic agonist, NMDA (Sigma, Poole, UK), were assessed by central injections of NMDA (200nl,10mM in aCSF) at CT 14 in both young (n = 11) and aged (n = 11) animals. Vehicle controls consisted of 200nl of artificial cerebral spinal fluid (aCSF (pH 7.4): 125.2 mM NaCl, 3.8 mM KCl, 1.2 mM KH2PO4, 1.8 mM CaCl2, 1 mM MgSO4, 24.8 mM NaHCO3, 10 mM D-Glucose). Finally, to investigate the role of the NR2B receptor subunit in photic resetting, both groups of young (n = 14) and old (n = 14) mice were exposed to a sub-saturating white light (150 lux) at CT14, preceded with central administration of either the NR2B antagonist, ifenprodil (200nL, 6.1μM in 10% dimethylsulfoxide (DMSO), Sigma UK), or a 10% DMSO (200nl) vehicle control at CT13.5.
Data were analysed blind to condition by extrapolation lines eye-fitted through the onset of 7 days of wheel activity before the light pulse. Post pulse regression lines were fitted using onsets from 7 days of activity out of the 10 days recorded, as the first 3 days after the light pulse were excluded as transients. The difference between the extrapolation lines, on the day of the stimulus, was measured in order to calculate behavioural phase shifts.
2.4. Pupillometry
Pupillometry (University of Kent) was conducted as previously described (Lucas, 2003) on unanesthetized young (n= 8) and aged (n= 8) mice. Animals were stably entrained to a 12 hr:12 hr LD cycle (white fluorescent source, 350 lux) and recordings were restricted to between 4 and 7 hr after lights on (ZT 4-7). All experiments were preceded by 1 hr of dark adaptation. Pupillary responses were elicited with Ganzfeld light stimuli (Xe arc source, filtered with neutral density filters) applied to one eye, previously dilated with 0.1% atropine allowing consensual pupil constriction to be recorded with a CCD camera. Ten seconds of darkness separated pre-treatment and test stimuli. Pupil area was measured using ImageJ (NIH, USA) (Schneider et al., 2012) analysis software and expressed relative to its size in the 3 seconds prior to light onset. As an exclusions criterion, animals presenting with cataracts (detected under infrared light) would not be included in the analysis. In the sampling population used in this study no animals exhibited signs of cataracts.
2.5 Electrophysiology
Young (n=6) and old (n=7), adult, male C57BL/6J mice (University of Glasgow) were housed in LD 12:12. Zeitgeber time (ZT) was defined as ZT 12 being the projected time of lights off in the animal room. Mice were administered an overdose of halothane anaesthesia and decapitated during the phases when this manipulation does not induce phase shifts (Gillette, 1986), between ZT 2 and ZT 5. Hypothalamic slices (500 μm) containing the SCN were placed in a gas-fluid interface slice chamber (Medical Systems BSC with Haas top), continuously bathed (1 ml/min) in artificial cerebrospinal fluid (aCSF). aCSF (pH 7.4) was supplemented with an antibiotic (gentamicin, 0.05 gm/l) and a fungicide (amphotericin, 2 mg/l) and maintained at 34.5°C. Warm, humidified 95% oxygen: 5% carbon dioxide was continuously provided.
Extracellular single-unit activity of SCN cells was detected with glass micropipette electrodes filled with aCSF, advanced through the slice using a hydraulic microdrive. The electrode was placed into regions of the SCN at random, alternating between the left and right SCN. The signal was fed into an amplifier for further amplification and filtering, and was continuously monitored by an oscilloscope and audio monitor. Firing rate and interspike interval data were analysed using Spike 2 (Cambridge Electronic Design, Cambridge, UK) data acquisition software and a customised program for calculation of descriptive statistics. The average spontaneous firing rate and the ZT for each single unit encountered was recorded for 4–5 min by an experimenter blind to all treatments.
N-Methyl-D-aspartate (NMDA, Sigma Aldrich, Dorset, UK), was dissolved in aCSF (Biello et al., 1997) and applied at a concentration of 10μM as a 200-nl microdrop to the SCN area of the slice. Microdrops were administered using a Hamilton 1-μl syringe on the same day as slice preparation at ZT 14, with ZT 12 being defined as the animal's activity onset in vivo prior to SCN removal. Drugs were warmed to 34.5°C. Recordings were performed for approximately 20h during ZT 0–20 of the second 24 h in vitro. Slices with less than four cells recorded during any 1 h period were not included in the analysis.
Initially, the average firing rate of each cell recorded from one slice was plotted against the ZT of the recording. Slices without significant differences across firing rate data grouped into 1 h (ZT) bins (p < 0.05; ANOVA) were not used for further analysis. If there were significant differences, data were smoothed by 1 h running means with a 15 min lag. The time corresponding to the maximum of the smoothed data was used as the time of the peak firing. Phase shifts were measured relative to the average time of peak firing of the age appropriate control slices treated with aCSF at ZT 14.
2.6. Real Time PCR
Young and aged mice (University of Kent and Smith College) entrained under a 12:12 LD cycle were sacrificed by cervical dislocation at ZT 6 (6 hours after lights on, young n= 7, old n= 7) and ZT 18 (6 hours after lights off, young n= 7, old n=7), to give midday and midnight time points respectively. Bendova et al. (2012) previously demonstrated circadian variation in both mRNA and protein expression of the NR2B subunit in the rat SCN. Protein levels were highest during the night at CT 18 and lower during the subjective day. Coronal brain sections containing the SCN were collected between ZT 5.5-ZT 6.5 and between ZT 17.5-ZT 18.5 using a vibratome. The SCN was isolated from these sections using a punch tool and stored in RNAlater (Sigma Aldrich, Dorset, UK) at -80°C until processed. Total RNA was isolated from SCN extracts using TRI Reagent (Applied Biosystems, UK). 100-500ng total RNA was reverse-transcribed using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche, UK). 20μL reactions were run in triplicate in a 96 well white-coloured plate containing 10μL 2× SYBR Green Mix (Roche Applied Sciences, Sussex, UK), 1μL 0.5μM forward primer, 1μL 0.5μM reverse primer, 7μL nuclease-free water and 1μL sample cDNA on a LightCycler 480 Real Time PCR machine (Roche Applied Sciences, Sussex, UK).
Primer sequences for RT-PCR identification, as reported by Slattery et al. (2006): NR1f:GCTGTACCTGCTGGACCGCT, NR1r:GCAGTGTAGGAAGCCACTATGATC, NR2Af:GCTACGGGCAGACAGAGAAG, NR2Ar:GTGGTTGTCATCTGGCTCAC, NR2Bf:GCTACAACACCCACGAGAAGAG, NR2Br:GAGAGGGTCCACGCTTTCC, 18S-rRNAf:CGCCGCTAGAGGTGAAATTC, 18S-rRNAr:CGAACCTCCGACTTTCGTTCT.
Reactions were pre-incubated at 95°C for 5 minutes before 55 cycles of 95°C for 10 seconds, 60°C for 10 seconds and 72°C for 10 seconds. Fluorescent detection after each cycle occurred at 82°C. Relative quantification was determined using the 2-ΔΔCT method described previously by (Livak and Schmittgen, 2001).
2.7 Power Analysis
We have previously reported the effects of saturating and sub-saturating light exposure at CT 14 on behavioural phase delays in young C57Bl/6J mice (Bonsall and Lall, 2013). A post hoc analysis showed that sub-saturating light had a large effect (Cohen's d = >4) on behavioural phase shifts. Similarly, others have demonstrated that intracranial volume delivery of NMDA at CT 14 causes significant behavioural phase delays with a large effect size (Cohen's d = 2) (Mintz et al., 1999). We predicted that aged mice will show a significant, but smaller effect in response to light or NMDA exposure. Using G*Power (Faul et al., 2009, 2007), a priori analysis indicated that more conservative effects of Cohen's d = 1.4 at >80% power would require group sizes of n=10 to detect significance at alpha = 0.05.
A post hoc power analysis of our previous electrophysiology study showed NMDA has a large effect on both amplitude and phase shifting of SCN firing in both young (Cohen's d = >8) and aged (Cohen's d = >9) mice (Biello, 2009). A priori power analysis predicting a smaller effect size, Cohen's d = 1.8 at >80% power would be sufficient to detect significant differences (alpha = 0.05) with groups sizes of n=6.
2.8 Statistical Analysis
All results are reported as mean ± standard error of the mean (SEM). For all experiments, statistical significance was determined using GraphPad Prism software (V.6., San Diego, CA) and defined as p < 0.05. All data groups were tested for, and successfully passed (p> 0.05), normality scoring using the D'Agostino & Pearson omnibus normality test. Behavioural phase shifts to light were analysed using a repeated measures two-way analysis of variance (ANOVA) (light intensity × age as factors) followed by Tukey-Kramer multiple comparisons post hoc test. Behavioural phase shifts to NMDA/Ifenprodil were analysed using a repeated measures two-way ANOVA (treatment × age as factors) followed by Tukey-Kramer multiple comparisons test. Pupillometry data were analysed using curve fit analysis applying the Least squares method and the extra sum-of-squares F test was used for comparison purposes.
Significant differences between groups (p < 0.05) were determined by a one-way ANOVA followed by a Tukey-Kramer post hoc test correcting for multiple comparisons, or a t-test. Differences between treatment conditions were determined within each age group, comparing peak time in age appropriate untreated slices with drug treatments. All results are reported as mean ± standard error of the mean (SEM). For the electrophysiology data, treatment effects were analysed within age groups by one-way ANOVA followed by Tukey-Kramer multiple comparisons, or a t-test. Differences between treatment conditions were determined within each age group, comparing peak time in age appropriate untreated slices with drug treatments. Real Time PCR data was analysed by comparison of ΔCT values by two-way ANOVA (ZT time × age as factors) with Tukey-Kramer multiple comparisons post hoc test for each subunit.
3. Results
3.1. Light-driven behavioural clock resetting is attenuated in aged mice
The effects of both dim (30 lux) and bright (300 lux) fluorescent white light were studied on their ability to reset behavioural circadian rhythms in young and old mice. Young mice displayed a characteristic intensity-dependent response with greater phase shifts observed on exposure to a 300 lux stimulus (-150 ± 5 minutes (mean ± SEM); n= 14, two-way ANOVA, F(3,39) = 105.3, p< 0.0001, Fig.1A) relative to 30 lux (-80 ± 4 minutes, n=14). A similar trend was observed in older mice where an order of magnitude increase in light intensity resulted in shifts significantly greater than those to a dim light, with resulting shifts of -74 ± 6 (n = 14, p<0.0001) and -35 ± 3 (n= 14) minutes respectively. However, under both photic conditions, younger mice demonstrated significantly greater phase shifts (p< 0.0001) relative to the aged with approximately 50% increases in response to dim and bright light pulses. Two-way ANOVA indicated that age accounted for 84.31% of the variance in our data set relative to light intensity which accounted for 5.28% (F(13,39) = 1.52, p>0.05).
Figure 1.
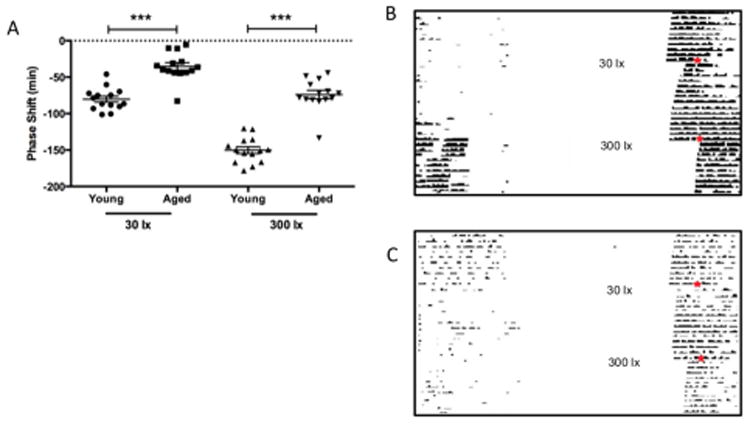
Circadian behavioural resetting to light in aged mice. Older mice (n= 14) show attenuated behavioural responses to both saturating (300 lx) and sub-saturating (30 lx) light stimuli compared to younger animals (p<0.001, n= 14)) (A). Young and aged mice exhibit an intensity-dependent response to light, with greater phase shifts observed in those mice receiving the higher intensity stimulus. Representative actograms displaying both responses to 30 and 300 lx in a young (B) and aged (C) mouse model; day and time of stimulus is indicated by ✶.
3.2. Reduced pupillary light reflex sensitivity in old mice
The observed diminishing effects of light at a behavioural level is interesting; however, it is unclear if this effect was due to critical changes across the photic transduction pathway or at the level of the SCN. In order to dissect out possible contributing factors, we began by looking at retinal sensitivity i.e. the point at which photic signals are initially interpreted. The pupillary light reflex serves as an excellent, high resolution indicator of direct photoreceptor decoding of light (Lucas et al., 2012). Dark adapted mice were exposed to a range of light intensities from very bright, 14 log photons/cm2/s to diminishing light in neutral density stops up to a total of 7 log units. Once plotted, we analysed our data by applying a Least squares curve fit extrapolation (R2 =0.95 for young animals, R2 = 0.87 for old) and comparison using the Extra sum-of-squares F test (Fig. 2A). We found that there was a significant shift between the two groups, with younger mice having a greater sensitivity, LogEC50= 9.519 in comparison to older animals, LogEC50= 9.883 (F(1,116) = 5.494, p<0.05, n= 8).
Figure 2.
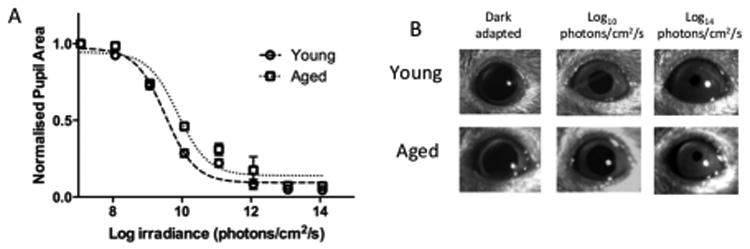
Irradiance response curves for ‘steady state pupil size’ in young and old mice. (A) Consensual pupil constrictions to white light demonstrated a shift in sensitivity between young (n=8) and old mice (n=8). Following curve fit analysis (A) the LogEC50 was greater in the aged group (p<0.05); thus, aged animals show an overall decrease in sensitivity to light relative to their younger counterparts. Representative pupil images (B) show stable dark-adapted pupil sizes in both young and aged animals, with decreasing pupil areas when exposed to intermediate (Log10 photons/cm2/s) and bright (Log14 photons/cm2/s) irradiances.
3.3. Glutamatergic signalling is impaired in the SCN of old mice
In order to assess the contribution of the glutamatergic-signalling cascade in the attenuation of light-driven clock resetting, we targeted the NMDA receptor as a likely candidate. Central administration of NMDA (10mM) at the level of the SCN in vivo resulted in significantly greater behavioural phase shifts in young mice compared to older counterparts with delays of 54 ± 9 (n= 9, t= 3.38, p< 0.01) and 12 ± 9 (n = 11, Fig. 3A) minutes respectively.
Figure 3.
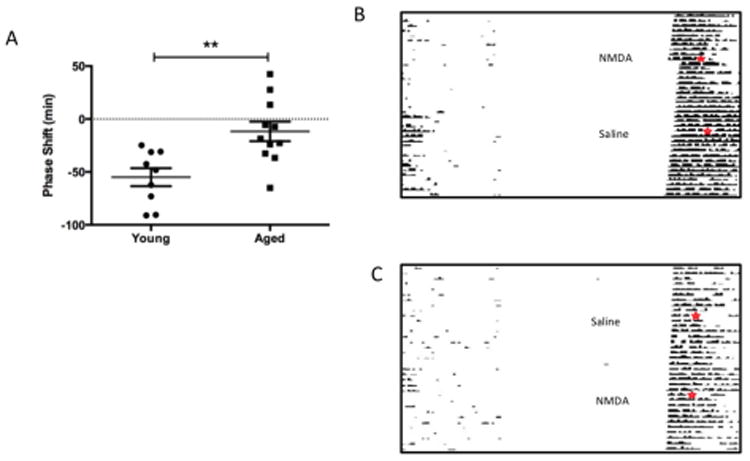
Circadian behavioural resetting to NMDA in aged mice. Central injections of NMDA reset behavioural circadian rhythms in young animals (A, B, p<0.01, n= 9), however had very little impact in older mice (A, B, n= 11). NMDA microinjections, in vivo, result in behavioural clock resetting in a similar manner to light. This response was significantly reduced in older animals (p<0.01, A). Representative actograms for a young (B) and aged (C) mouse undergoing either NMDA or saline treatments; day and time of injections is indicated by ✶.
The behavioural effects of NMDA were further isolated to the SCN through coronal slice recordings of spontaneous electrical firing rhythms in young and aged mice. Young mice displayed shifts in SCN firing peak rhythms of -232 ± 7 minutes (n= 6, Fig. 4A) from controls following application of NMDA. However, old mice only displayed shifts of -100 ± 14 minutes (n=7), significantly less than younger mice (p< 0.0001, t= 8.084). In addition, there was also an observed decrease in the peak amplitude of firing in the aged samples (n=7; 4.07 ± 0.21 Hz (mean ± SEM)) as compared with the young (n=6; 6.02 ± 0.15 Hz, p <0.01), as previously reported (Biello, 2009; Nakamura et al., 2011)
Figure 4.
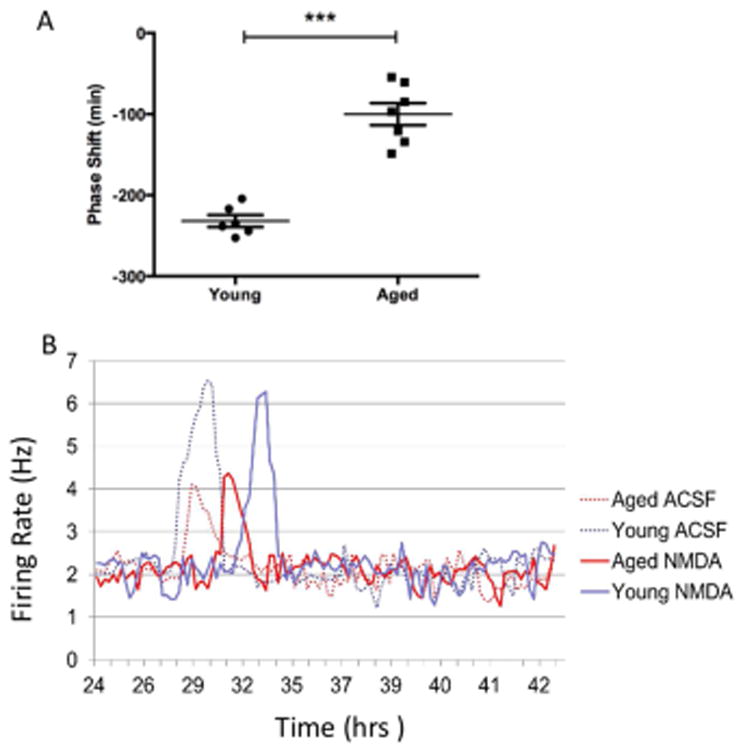
Cellular firing rhythms in SCN brain slices from young and aged mice. (A) Electrophysiological recordings from isolated aged SCN brain slices displayed attenuated shifts in the peak firing rhythms following exposure to NMDA in comparison with preparations from younger animals (p<0.001, n= 6 young mice; n= 7 aged mice). (B) There was also an observed decrease in the peak amplitude of firing in the aged samples (n=7; 4.07 ± 0.21 Hz (mean ± SEM)) relative to recordings taken from young mice (n=6; 6.02 ± 0.15 Hz, p <0.01). Here we show the frequency of SCN cells firing rates represented by a 1-h running mean with a 15-min lag over time for four individual slices recorded from young and aged mice.
3.4. NR2B expression is dramatically diminished in old mice
Having found significant alterations in NMDA signalling, both behaviourally and centrally at the level of the SCN, we went on to investigate if changes in specific NMDA subunit expression were contributing to a decline in this response. Age-dependent variations in day (ZT 6) and night (ZT 18) expression of the NMDA receptor subunits NR1, NR2A and NR2B in the SCN were assessed using real time PCR.
Analysis of the NR1 subunit by two-way ANOVA showed a significant effect of time (F(1,16) = 15.33, p = 0.0012) and age (F(1,16) = 12.08, p = 0.0031) with no significant interaction (F(1,16) < 0.001, p = 0.987, n=3-7). There was a significant interaction between time and age (F(1,15) = 10.89, p = 0.0049) in the expression of NR2A within the SCN, with no individual effects of either factor (time: F(1,15) = 1.634, p = 0.221, age: F(1,15) = 0.0023, p = 0.962, n=3-7). Age-dependent multiple comparisons showed significantly higher levels of NR1 (p = 0.047) and NR2A (p = 0.01) in the aged SCN during the day (Fig. 5A). However, the NR2B subunit showed a significantly aged-dependent decrease in expression (F(1,16) = 54.40, p < 0.001) that was not dependent on time of day (F(1,16) = 0.818, p = 0.379) or an interaction of factors (F(1,16) = 0.896, p = 0.358, n=3-7). During the night, no significant change was observed in either the NR1 or NR2A subunits between the young and old, but the NR2B exhibited lower expression in aged mice (p < 0.001, Fig. 5B).
Figure 5.
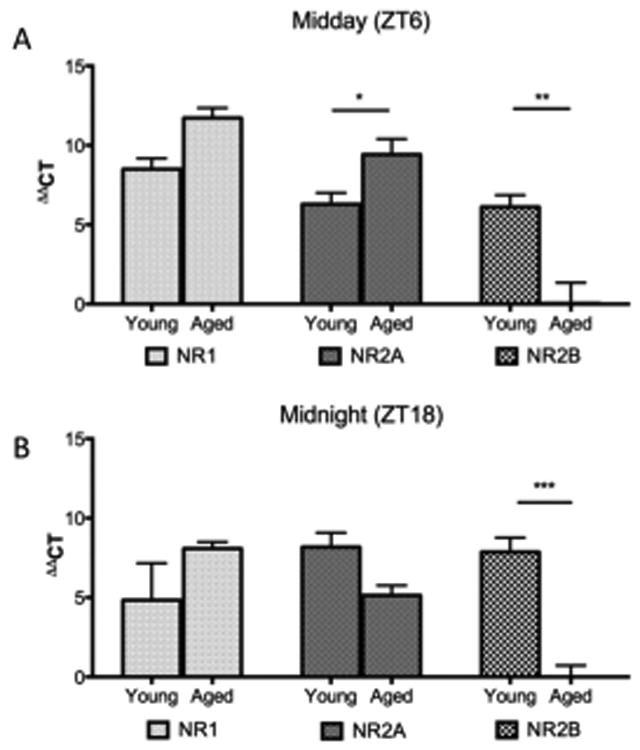
Diurnal expression of NMDA receptor subunits in the SCN. Relative expression (ΔΔCT) of NMDA subunits from SCN tissue were assessed at two time points by RT-PCR, (A) midday (ZT 6) and (B) midnight (ZT 18). Midday samples showed age-dependent changes in the expression of NR2A and NR2B (p<0.05, n= 3-7). Midnight samples showed an age-dependent decline in NR2B expression in aged mice relative to the younger cohort (p<0.001).
3.5. Inhibition of the NR2B subunit attenuates behavioural phase shifts to light in young, but not old mice
The reduction in expression of the NR2B subunit in the SCN of old mice strongly suggested that it could be a key contributor in the behavioural changes observed in light-induced circadian responses of aged animals. In order to assess this hypothesis, light pulses were presented to young and old mice pre-treated with the NR2B antagonist, Ifenprodil (6.1μM). Control groups consisting of central vehicle injections followed by a light pulse resulted in reduced phase shifts in older animal's relative to younger mice, with delays of 37 ± 3 (mean ± SEM, n= 10) and 78 ± 3 (n=14) minutes respectively (p<0.001). Following a two-way ANOVA, it was found that there was significant interaction between age and Ifenprodil treatment (F(1,44) = 44.78, p<0.0001). Inhibition of the NR2B subunit using the antagonist Ifenprodil reduced the resetting effects of light by approximately 60% in young animals when compared to light with vehicle administration in this group (F(1,44) = 13.16, p<0.0001, Fig. 6A) with resulting shifts of 30 ± 2 minutes and 78 ± 3 minutes (n= 14) respectively. Interestingly, injections of Ifenprodil prior to light stimulation in aged mice did not alter the magnitude of the response when compared with light plus vehicle treatments, with delays of 51 ± 7 minutes and 37 ± 6 minutes (n=10) respectively. Although in this experiment we saw unexpected scatter of results, even with the level of variation in the vehicle-treated aged group we were still powered for a difference of ± 21 min. Yet the results from the drug-treated group gives no indication of a difference in response similar to the decreased shift we observed in the young mice, and our statistical tests (both the planned parametric as well as a post-hoc non-parametric Kruskal-Wallis test) showed no significant difference between the groups.
Figure 6.
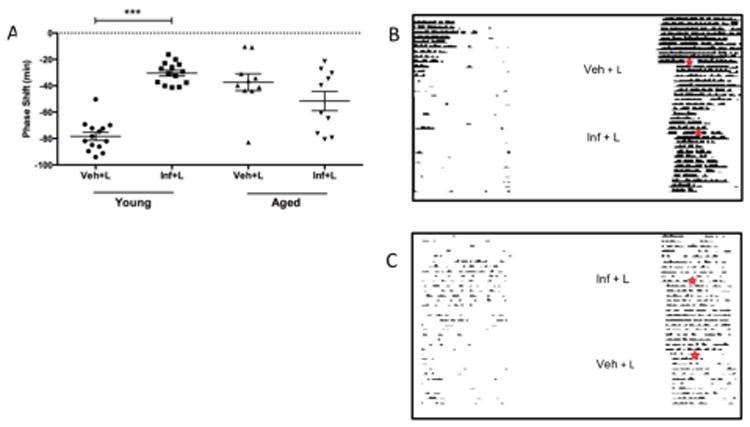
Behavioural resetting mediated through the NR2B subunit. (A) Administration of the NR2B antagonist Ifenprodil, via a cannula aimed at the SCN, attenuated the resetting effects of light (150 lux) in young mice (p<0.001, n= 14). However, there was no level of inhibition observed in aged animals (p>0.05, n= 10). Representative locomotor records for young (B) and aged (C) mice undergoing vehicle (Veh), light (L) and or NR2B antagonist (Inf) treatment; day and time of stimulus is indicated by ✶.
4. Discussion
The data from this study provide evidence, for the first time, that shows circadian photoentrainment is impaired in aged animals through changes in SCN glutamatergic input. We have demonstrated that older mice show reduced circadian resetting to light. With only a modest change observed in pupillary light sensitivity, this effect most likely resides at the level of the SCN, where NMDA application, both in vivo and in vitro, resulted in attenuated phase shifts in aged animals. In addition, we have established that aged mice show dramatically decreased SCN expression of the NMDA NR2B subunit. Finally, we tested the functional importance of the NR2B subunit in mediating phase shifts to light in vivo. The NR2B selective antagonist, Ifenprodil, was able to inhibit clock resetting in young animals, however no effect was observed in aged mice. Taken together, our data links the reduction in expression of the NR2B subunit to the diminishing sensitivity of the SCN to light and the resultant impact on photoentrainment.
The most common attribute associated with normal aging is that of alterations in sleeping routines (Carrier et al., 2017; Feinberg et al., 1967; Ohayon et al., 2004). These typically manifest as reduced sleep duration and increased awakening frequency during the night (Klerman et al., 2004). Such changes can be observed in humans aged from as early as 20 years old and upwards (Carrier et al., 1997; Landolt et al., 1996; Luca et al., 2015). Older humans show advanced sleep phase (sleeping and waking earlier), greater disruption of sleep in response to circadian misalignment, and an increased prevalence of Shift Work Disorder (Duffy et al., 2015). Key circadian physiological markers that show misalignment include core body temperature, melatonin and cortisol secretions, urine production and higher-level behavioural functions such as awareness and cognition (Campbell and Dawson, 1992; Carrier et al., 1999; Czeisler et al., 1992; Duffy et al., 2016; Sharma et al., 1989; Silva et al., 2010; Valentinuzzi et al., 1997). Alterations in circadian photoentrainment, such as those presented in this study, are likely to be fundamental to these physiological misalignments associated with age. Here we present data showing alterations in light input signalling to the SCN, suggesting central nervous system changes likely contribute to such circadian desynchronization.
The aging eye is susceptible to a number of anatomical and functional changes. Yellowing/ opaqueness of the lens is common in the elderly and reduces transmission of short wavelengths of light (Rukmini et al., 2017). In addition, physiological changes at the retina due to alterations in cell morphology or death are also prevalent, either due to natural aging or disease, such as glaucoma (Lin et al., 2016). Thus, the eye presents as a potential candidate for limiting light input to the SCN. We assessed age related retinal function through the pupillary response to light in our animals, to determine if changes in sensitivity in the eye could explain the attenuated phase resetting to light. At the brightest light intensities, both young and old pupil constrictions were virtually identical, indicative of a fully functioning melanopsin-induced phototransduction pathway. Given that this photoreceptor is the most important for circadian clock resetting, it is unlikely that age related changes in the retina contribute to the behavioural deficits that we have reported (Freedman et al., 1999). The shift in pupillary sensitivity observed was mainly through responses to intermediate light (Fig.2a) which are largely associated with cone photoreceptors, but there is little evidence supporting the involvement of cones in circadian behavioural resetting (Lall et al., 2010). Humans show loss of melanopsin-immunoreactive retinal ganglion cells after 70 years of age, with loss of the dendritic arbor apparent after age 50 (Esquiva et al., 2017). Further, older people have a smaller pupil than younger controls, but pupillary response to light does not differ with age in people (Daneault et al., 2012). Thus, we cannot rule out a role of the aged eye as a contributor to the deteriorating photic input to the SCN; however, this is likely to have minimal effect on our results due to the higher, melanopsin-favoured irradiances used in our photoentrainment protocols.
Our electrophysiological recordings from SCN slices showed a decreased SCN firing amplitude in aged mice relative to young (Fig.4B), supporting previously published data (Biello, 2009; Farajnia et al., 2012; Nakamura et al., 2015, 2011). Individual SCN neurons do not appear to show loss of amplitude, but cellular coupling appears to be weakened, an effect that can be counteracted by strong light-dark cycles (Farajnia et al., 2014). Our research suggests that these critically important synchronizing effects of light on SCN cells are impaired with aging. The deficits observed in resetting to photic stimuli as mice age correspond with molecular changes within the SCN (Benloucif et al., 1997; Zhang et al., 1996). These include a reduction in the photic activation of c-fos mRNA or FOS protein, of the cyclic-AMP response element binding protein (CREB), and induction of Per1 (Kolker et al., 2003; Zhang et al., 1996). Our previous in vitro resetting work (Biello, 2009) suggested that these molecular changes were at least partially due to alterations at the NMDA receptor. We have further extended this to understand how changes in the NR2B subunit contribute to a portion of observed changes in responses to light with age.
The NMDA receptor presents as a multimeric protein complex consisting of a NR1 subunit and a varying number of NR2 subtypes (Cull-Candy et al., 2001; McBain and Mayer, 1994). The NR2 subunit defines the pharmacological properties of the receptor. For instance, a NR1 and NR2B combination displays slower kinetic properties with high permeability to Ca2+ ions (McBain and Mayer, 1994). Such attributes are likely to be favourable for relaying photic information to the SCN, allowing the greatest probability of cellular excitation through the inward flow of Ca2+ ions. In addition, there is also evidence of rapid switching of subunits, for example NR2B and NR2A exchanges have been found in the visual cortex following light exposure (Quinlan et al., 1999). Our analysis of NMDA receptor subunit expression revealed variation in expression of NR1, NR2A and NR2B across midday and midnight as well as between young and old mice. Our most striking finding was the significant decrease in NR2B expression in the old animals at both times of the day. Thus, this would suggest that aged animals have a higher proportion of NR1-NR2A receptor structures; potentially impacting glutamatergic transmission to the SCN. Further, there is also some suggestion of a circadian subunit expression profile, with an overall upregulation in all subunits during the night in the young, with NR2B rhythmicity previously reported (Bendova et al., 2012; Wang et al., 2008). However, this warrants further detailed investigation and is beyond the scope of the present study.
Variations in receptor expression at the mRNA level do not always necessarily translate to physiological relevance. Thus, to this end, we tested to see if the reduced expression in NR2B was physiologically significant. Ifenprodil is an antagonist that binds to NMDA receptors containing the NR2B subunit and shows a 200-fold preference for this configuration over other permutations (Neyton and Paoletti, 2006; Williams, 2001, 1993). Central injection of ifenprodil directly onto the SCN in vivo attenuated light-induced phase shifts in the young, showing a role of NR2B-containing receptors in mediating light signalling within the SCN. Significantly, there was an absence of any effect of the antagonist in aged animals; thus, affirming the NR2B subunit as a likely candidate for the decline in glutamate signalling to the SCN in old mice.
Our study was undertaken in male C57BL/6J mice, which is an established model for circadian research. Circadian behavioural measures in mice are influenced by hormonal status (Krizo and Mintz, 2015). A recent meta-analysis concluded that male mice can show increased variability in behavioural measures, especially so when group-housed (Prendergast et al., 2014). While female mice show increased variability in behavioural activity across the 4 day estrus cycle, males show minimal day to day variations and increased variation within a day (Smarr et al., 2017). Retina, SCN and CNS target regions of the SCN all express receptors for gonadal hormones, indicating the possibility for multiple levels of interactions with sex (Bailey and Silver, 2014). There is emerging evidence that suggests sex differences in the regulation of both sleep and circadian rhythms through age, predominantly due to sex hormone expression (Carrier et al., 2017; Kuljis et al., 2013). Thus, future animal studies would benefit from including sex as a significantly important variable in assessing the impact of aging on maintenance and regulation of circadian rhythms.
We designed our studies of photoentrainment using short light pulses, allowing us measures of changes with age and following administration of pharmacological agents. Our mice were housed in constant darkness, a condition that highlights changes with age that can be masked by LD cycles (Nakamura et al., 2015). A limitation of this approach is that while mice show robust phase delays to short light pulses, phase advances are smaller, being less than an hour even in young control mice (Spoelstra et al., 2004). Studies using shifts of full light: dark cycles have shown that older mice show reduced resetting to advances of the full LD cycle (Valentenuzzi et al., 1997; Leise et al., 2013), leading us to anticipate that our current research findings extend to phase advances, but this should be explicitly tested in future studies.
5. Conclusion
In summary, we have found that the aging process impairs behavioural circadian clock resetting to light, an essential component for environmental photoentrainment. Further, through electrophysiological recordings and real-time expression analysis of SCN tissue, we have narrowed down the NMDA NR2B subunit as a significant key contributor to age-induced decline in light sensitivity. Most significantly, we have shown this to be the case in vivo, thus providing functional evidence inferred from our molecular and cellular data. Overall, in the aging mammalian circadian system it would appear that the alterations in glutamatergic drive to the SCN through the NMDA receptor play a fundamental role in the challenges faced by the circadian clock in generating a stable and robust photoentrained rhythm. Finally, by establishing the significant changes in NMDA receptor configuration through age and the impact on circadian synchronisation we have uncovered a novel therapeutic target for the potential treatment of circadian misalignment in aged individuals.
Behavioural circadian clock resetting to light is impaired in aged animals
NMDA is less effective in driving phase shifts of circadian rhythms with age
Ionotropic glutamate receptor subunit re-organisation is evident in older mammals
Reduced NR2B expression in the suprachiasmatic nucleus impacts circadian function
Acknowledgments
This work was supported, in part, by funding from the following sources: Royal Society UK (RG100842) to GSL, Research grant award from the University of Kent to DRB and GSL, NIH EARDA (Smith College) and NIH NIA 5P01AG009975-18 both to MEH. Daily care of animals was performed by Charles River (UK) for the University of Kent, by the Joint Animal Facility for the University of Glasgow, and by the Smith College Animal Care Facility.
Footnotes
Disclosure statement: All authors report no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35:111–139. doi: 10.1016/j.yfrne.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendová Z, Sládek M, Svobodová I. The expression of NR2B subunit of NMDA receptor in the suprachiasmatic nucleus of Wistar rats and its role in glutamate-induced CREB and ERK1/2 phosphorylation. Neurochem Int. 2012;61:43–47. doi: 10.1016/j.neuint.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Masana MI, Dubocovich ML. Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res. 1997;747:34–42. doi: 10.1016/s0006-8993(96)01182-1. [DOI] [PubMed] [Google Scholar]
- Biello SM. Circadian clock resetting in the mouse changes with age. Age (Omaha) 2009;31:293–303. doi: 10.1007/s11357-009-9102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biello SM, Golombek Da, Schak KM, Harrington ME. Circadian phase shifts to neuropeptide Y In vitro: cellular communication and signal transduction. J Neurosci. 1997;17:8468–75. doi: 10.1523/JNEUROSCI.17-21-08468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsall DR, Lall GS. Protein kinase C differentially regulates entrainment of the mammalian circadian clock. Chronobiol Int. 2013;30 doi: 10.3109/07420528.2012.741170. [DOI] [PubMed] [Google Scholar]
- Campbell S, Dawson D. Aging young sleep: a test of the phase advance hypothesis of sleep disturbance in the elderly. J Sleep Res. 1992;1:205–210. doi: 10.1111/j.1365-2869.1992.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Carrier J, Monk TH, Buysse DJ, Kupfer DJ. Sleep and morningness-eveningness in the “middle” years of life (20-59 y) J Sleep Res. 1997;6:230–237. doi: 10.1111/j.1365-2869.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- Carrier J, Monk TH, Reynolds CF, 3rd, Buysse DJ, Kupfer DJ. Are age differences in sleep due to phase differences in the output of the circadian timing system? Chronobiol Int. 1999;16:79–91. doi: 10.3109/07420529908998714. [DOI] [PubMed] [Google Scholar]
- Carrier J, Semba K, Deurveilher S, Drogos L, Cyr-Cronier J, Lord C, Sekerovick Z. Sex differences in age-related changes in the sleep-wake cycle. Front Neuroendocrinol. 2017;40:66–85. doi: 10.1016/j.yfrne.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Ralph MR, Menaker M. Do NMDA receptors mediate the effects of light on circadian behavior? Brain Res. 1990;523:117–20. doi: 10.1016/0006-8993(90)91643-u. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, Sánchez R, Ríos CD, Ronda JM. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- Daneault V, Vandewalle G, Hébert M, Teikari P, Mure LS, Doyon J, Gronfier C, Cooper HM, Dumont M, Carrier J. Does Pupil Constriction under Blue and Green Monochromatic Light Exposure Change with Age? J Biol Rhythms. 2012;27:257–264. doi: 10.1177/0748730412441172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea Ma, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Scheuermaier K, Loughlin KR. Age-Related Sleep Disruption and Reduction in the Circadian Rhythm of Urine Output: Contribution to Nocturia? Curr Aging Sci. 2016;9:34–43. doi: 10.2174/1874609809666151130220343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Zitting KM, Chinoy ED. Aging and circadian rhythms. Sleep Med Clin. 2015;10:423–434. doi: 10.1016/j.jsmc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Herron JM, Hill SA. Aging selectively suppresses vasoactive intestinal peptide messenger RNA expression in the suprachiasmatic nucleus of the Syrian hamster. Mol Brain Res. 2001;87:196–203. doi: 10.1016/s0169-328x(01)00015-8. [DOI] [PubMed] [Google Scholar]
- Ebling FJP. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Esquiva G, Lax P, Pérez-Santonja JJ, García-Fernández JM, Cuenca N. Loss of melanopsin-expressing ganglion cell subtypes and dendritic degeneration in the aging human retina. Front Aging Neurosci. 2017;9:79. doi: 10.3389/fnagi.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, Deboer T, Rohling JHT, Meijer JH, Michel S. Aging of the suprachiasmatic clock. Neuroscientist. 2014;20:44–55. doi: 10.1177/1073858413498936. [DOI] [PubMed] [Google Scholar]
- Farajnia S, Michel S, Deboer T, vanderLeest HT, Houben T, Rohling JHT, Ramkisoensing A, Yasenkov R, Meijer JH. Evidence for Neuronal Desynchrony in the Aged Suprachiasmatic Nucleus Clock. J Neurosci. 2012;32:5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Koresko RL, Heller N. EEG sleep patterns as a function of normal and pathological aging in man. J Psychiatr Res. 1967;5:107–144. doi: 10.1016/0022-3956(67)90027-1. [DOI] [PubMed] [Google Scholar]
- Flores-Soto ME, Chaparro-Huerta V, Escoto-Delgadillo M, Vazquez-Valls E, González-Castañeda RE, Beas-Zarate C. Structure and function of NMDA-type glutamate receptor subunits. Neurologia. 2012;27:301–10. doi: 10.1016/j.nrl.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Muñoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–4. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Gillette MU. The suprachiasmatic nuclei: circadian phase-shifts induced at the time of hypothalamic slice preparation are preserved in vitro. Brain Res. 1986;379:176–181. doi: 10.1016/0006-8993(86)90273-8. [DOI] [PubMed] [Google Scholar]
- Gu C, Coomans CP, Hu K, Scheer FAJL, Stanley HE, Meijer JH. Lack of exercise leads to significant and reversible loss of scale invariance in both aged and young mice. Proc Natl Acad Sci. 2015;112:2320–2324. doi: 10.1073/pnas.1424706112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami F, Okamura H, Tamada Y, Maebayashi Y, Fukui K, Ibata Y. Loss of day-night differences in VIP mRNA levels in the suprachiasmatic nucleus of aged rats. Neurosci Lett. 1997;222:99–102. doi: 10.1016/s0304-3940(97)13355-9. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Davis JB, Duffy JF, Dijk DJ, Kronauer RE. Older people awaken more frequently but fall back asleep at the same rate as younger people. Sleep. 2004;27:793–798. doi: 10.1093/sleep/27.4.793. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging Alters Circadian and Light-Induced Expression of Clock Genes in Golden Hamsters. J Biol Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18:4767–4774. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizo JA, Mintz EM. Sex Differences in Behavioral Circadian Rhythms in Laboratory Rodents. Front Endocrinol (Lausanne) 2015;5:234. doi: 10.3389/fendo.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, Arnold AP, Colwell CS. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013;154:1501–12. doi: 10.1210/en.2012-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. NMDA receptor function during senescence: Implication on cognitive performance. Front Neurosci. 2015;9:1–15. doi: 10.3389/fnins.2015.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Güler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, Borbély AA. Effect of age on the sleep EEG: Slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–212. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- Leise TL, Harrington ME, Molyneux PC, Song I, Queenan H, Zimmerman E, Lall GS, Biello SM. Voluntary exercise can strengthen the circadian system in aged mice. Age (Omaha) 2013;35:2137–2152. doi: 10.1007/s11357-012-9502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JB, Tsubota K, Apte RS. A glimpse at the aging eye. npj Aging Mech Dis. 2016;2:16003. doi: 10.1038/npjamd.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luca G, Haba Rubio J, Andries D, Tobback N, Vollenweider P, Waeber G, Marques Vidal P, Preisig M, Heinzer R, Tafti M. Age and gender variations of sleep in subjects without sleep disorders. Ann Med. 2015;47:482–491. doi: 10.3109/07853890.2015.1074271. [DOI] [PubMed] [Google Scholar]
- Lucas RJ. Diminished Pupillary Light Reflex at High Irradiances in Melanopsin-Knockout Mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Lall GS, Allen AE, Brown TM. How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Prog Brain Res. 2012;199:1–16. doi: 10.1016/B978-0-444-59427-3.00001-0. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Sousa N, Santer RM, Paula-Barbosa MM, Gundersen HJG. Age and sex do not affect the volume, cell numbers, or cell size of the suprachiasmatic nucleus of the rat: An unbiased stereological study. J Comp Neurol. 1995;361:585–601. doi: 10.1002/cne.903610404. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Declines in mRNA Expression of Different Subunits May Account for Differential Effects of Aging on Agonist and Antagonist Binding to the NMDA Receptor. J Neurosci. 2000;20:1666–1674. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon Maurice M, Carskadon Mary A, Guilleminault Christian, Vitiello Michael V. Meta-Analysis of Quantitative Sleep Parameters From Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values Across the Human Lifes pan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Maynard ML, Zele AJ, Kwan AS, Feigl B. Intrinsically photosensitive retinal ganglion cell function, sleep efficiency and depression in advanced age-related macular degeneration. Investig Ophthalmol Vis Sci. 2017;58:990–996. doi: 10.1167/iovs.16-20659. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci. 1999;19:5124–30. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, Colwell CS, Block GD. Age-Related Changes in the Circadian System Unmasked by Constant Conditions. eNeuro. 2015;2 doi: 10.1523/ENEURO.0064-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. Age-Related Decline in Circadian Output. J Neurosci. 2011;31:10201–10205. doi: 10.1523/JNEUROSCI.0451-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott Ma, Perry EK, Perry RH, Court Ja. [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging. Brain Res. 1992;588:277–286. doi: 10.1016/0006-8993(92)91586-4. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA. 1999;96:12876–80. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rukmini AV, Milea D, Aung T, Gooley JJ. Pupillary responses to short-wavelength light are preserved in aging. Sci Rep. 2017;7:43832. doi: 10.1038/srep43832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satinoff E, Li H, Tcheng TK, Liu T, McArthur AJ, Medanic M, Gillette MU. Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones? Am J Physiol. 1993;265:R1216–R1222. doi: 10.1152/ajpregu.1993.265.5.R1216. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Palacios-Bois J, Schwartz G, Iskandar H, Thakur M, Quirion R, Nair NPV. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry. 1989;25:305–319. doi: 10.1016/0006-3223(89)90178-9. [DOI] [PubMed] [Google Scholar]
- Silva EJ, Wang W, Ronda JM, Wyatt JK, Duffy JF. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep. 2010;33:481–490. doi: 10.1093/sleep/33.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery JA, Page AJ, Dorian CL, Brierley SM, Blackshaw LA. Potentiation of mouse vagal afferent mechanosensitivity by ionotropic and metabotropic glutamate receptors. J Physiol. 2006;577:295–306. doi: 10.1113/jphysiol.2006.117762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr BL, Grant AD, Zucker I, Prendergast BJ, Kriegsfeld LJ. Sex differences in variability across timescales in BALB/c mice. Biol Sex Differ. 2017;8:7. doi: 10.1186/s13293-016-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelstra K, Albrecht U, van der Horst GTJ, Brauer V, Daan S. Phase Responses to Light Pulses in Mice Lacking Functional per or cry Genes. J Biol Rhythms. 2004;19:518–529. doi: 10.1177/0748730404268122. [DOI] [PubMed] [Google Scholar]
- Wang LM, Schroeder A, Loh D, Smith D, Lin K, Han JH, Michel S, Hummer DL, Ehlen JC, Albers HE, Colwell CS. Role for the NR2B subunit of the N-methyl-D-aspartate receptor in mediating light input to the circadian system. Eur J Neurosci. 2008;27:1771–1779. doi: 10.1111/j.1460-9568.2008.06144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Curr Drug Targets. 2001;2:285–98. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Wise PM, Cohen IR, Weiland NG, London ED. Aging alters the circadian rhythm of glucose utilization in the suprachiasmatic nucleus (middle age/diurnal rhythm/hypothalamus/estradiol/2-deoxyglucose) Neurobiology. 1988;85:5305–5309. doi: 10.1073/pnas.85.14.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff K. The role of photoreception and light intervention for sleep and neuropsychiatric disturbances in the elderly. Int Psychogeriatrics. 2015;27:1022–1029. doi: 10.2174/1567205014666170523095231. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273:R1957–64. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kornhauser JM, Zee PC, Mayo KE, Takahashi JS, Turek FW. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, Fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70:951–961. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]


