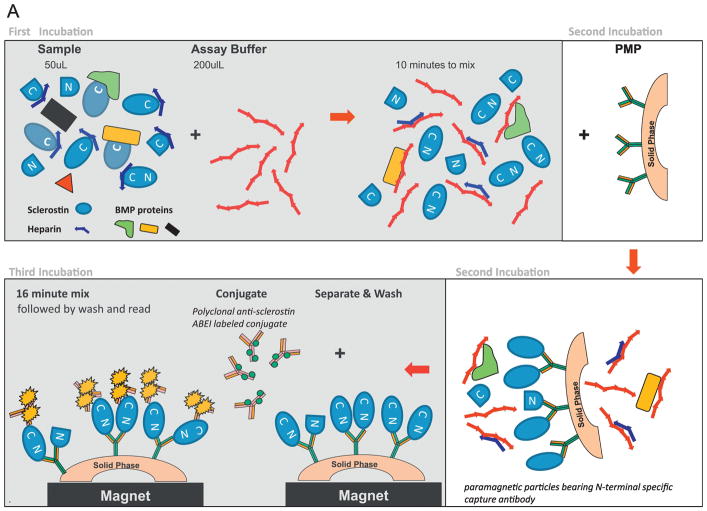
Figure 1. 1A. LIAISON sclerostin CLIA.
First incubation 1: 50 μl patient sample incubated with an assay buffer containing a basic polymer to dislodge anionic binding partners like heparin and BMP proteins than can occlude sclerostin’s C-terminal surface, making it more accessible to conjugate recognition. Second incubation: N-terminal specific murine monoclonal capture antibody coupled to paramagnetic particles is added. Upon binding sclerostin, it orients sclerostin such that the C-terminal portion of sclerostin is distal to the capture particle’s surface. A sequential separation and wash cycle is then performed to remove unbound sclerostin fragments and other non-specific serum/plasma proteins. Third incubation: Sandwich formation is achieved via addition of an ABEI-labelled polyclonal goat functionally anti-sclerostin C-terminal tail specific conjugate. Following a final separation and wash cycle, developer is added and emitted light photons [relative light units (RLU)] are measured and converted to pg/mL via a master standard curve. Total time to results is approximately 65 minutes.
1B. Specificity of the LIAISON sclerostin CLIA components overlaid upon a Clustal O(1.2.4) alignment of sclerostin and SOSTDC1 primary sequences. The capture monoclonal antibody is specific to a 16-mer located within the N-terminal tail (Gln1 to Ser56) of sclerostin. The tracer polyclonal antibody is conjugated to ABEI. Its functional C-terminal tail specificity is inferred from, and based upon, three observations: 1) The distal orientation of sclerostin’s C-terminal portion consequent to N-terminal capture specificity of the PMP; 2) SOSTDC1’s C-terminal portion (Gly117 to Ser206) that aligns with the C-terminal thrombin peptide of sclerostin (Gly99 to Tyr190) is twice as basic as its N-terminal portion (2.33fold [21.7%/9.2%] which is similar to sclerostin, whose C-terminal fragment is 2.23 fold [25%/11.2%] more basic than its N-terminal fragment; and 3) SOSTDC1, which has 47.1% direct homology to sclerostin’s post-thrombin digest C-terminal Loop2-Loop3 fragment domain (Gly99 to Arg149) and only 19.5% homology to the post-thrombin digest C-terminal tail domain (Phe150 to Tyr190), does not inhibit the assay when added to the third incubation step. Collectively these observations make it more likely that the conjugate’s specificity is predominantly towards the C-terminal tail.
1C. Primary attributes of sclerostin and SOSTDC1. Intact molecule: sclerostin (Gln1 to Tyr190) has 35.9% direct homology with SOSTDC1 (M1 to Ser206). Gly99 – Arg149 and Phe150 – Tyr190 comprise the Loop2–Loop3 and C-terminal portions of the C-terminal thrombin fragment, respectively. Percent basic (arginine/lysine) residue content of sclerostin secondary structural elements (i.e. Loops 1, 2 and 3) along with the N- and C-terminal thrombin fragments (and the aligned portions from SOSTDC1) demonstrate a similar relative basic aspect to the C-terminal portion of each molecule.