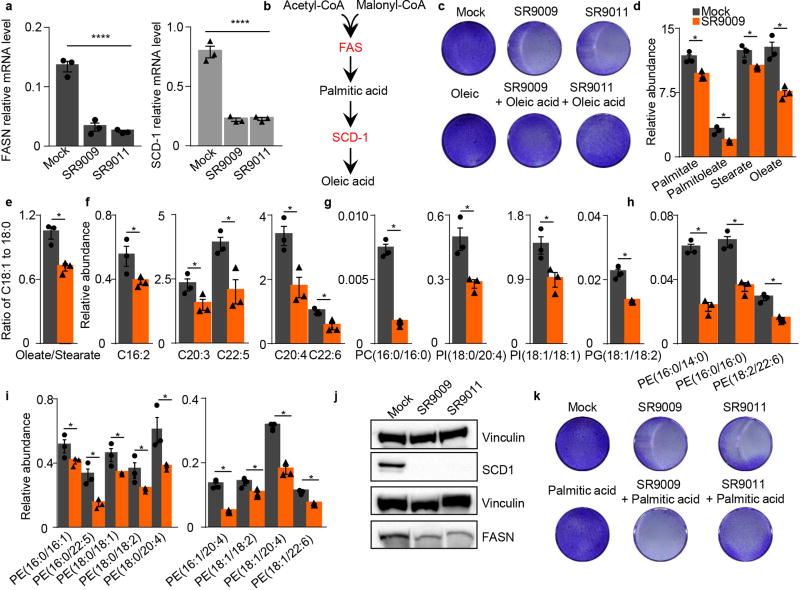
Extended Data Figure 4. REV-ERB agonists inhibit de novo lipogenesis.
a–b, REV-ERBs induces downregulation of FASN and SCD-1 mRNA as assayed by qRT-PCR (A172 glioblastoma cell line 48h, 20µM, FASN and SCD-1 (n= 3 biological independent samples ****P<0.0001); Also, FASN and SCD-1 protein levels (b) are reduced upon treatment. c–i REV-ERB agonists reduce free fatty acid (FFA) concentrations as quantified by LC-MS. c, Relative levels of FFAs that are the primary products of FASN (C16:0, C18:0) and SCD-1 (C16:1, C18:1) are lower in the SR9009 treated samples. d, the unsaturation index, the ratio oleate-to-stearate changes is decreased in the SR9009 treated sample, due to a larger decrease in monounsaturated oleate concentrations; e–f, Analysis of polyunsaturated fatty acids shows a consistent trend with lower levels of these fatty acids; g–i, decreases in FFA levels can affect the concentrations of phospholipids that contain those fatty acids. REV-ERB agonists treatment leads to a reduction of palmitate-containing phosphatidylcholine, arachidonic acid- and oleic acid-containing phosphatidylinositols, mono- and di-unsaturated phosphatidylglycerol, and h–i phosphatidylethanolamines; (A172 glioblastoma cell line 48h, 20µM, *P=0.05. j, Scheme illustrating metabolic products of FASN and SCD-1; k, Supplementation of oleic acid partially ameliorate REV-ERBs agonists’ cytotoxicity (20 µM, A172 4 days); l, Supplementation of palmitic acid does not impair REV-ERBs agonists’ cytotoxicity (20 µM, A172 4 days). All the data are plotted as mean ± s.e.m. P-value is calculated with one-way ANOVA in panel a, and with Mann–Whitney test one-tailed in the remaining panels. d–i n=3 biological independent samples. For gel source data, see Supplementary Fig. 1.