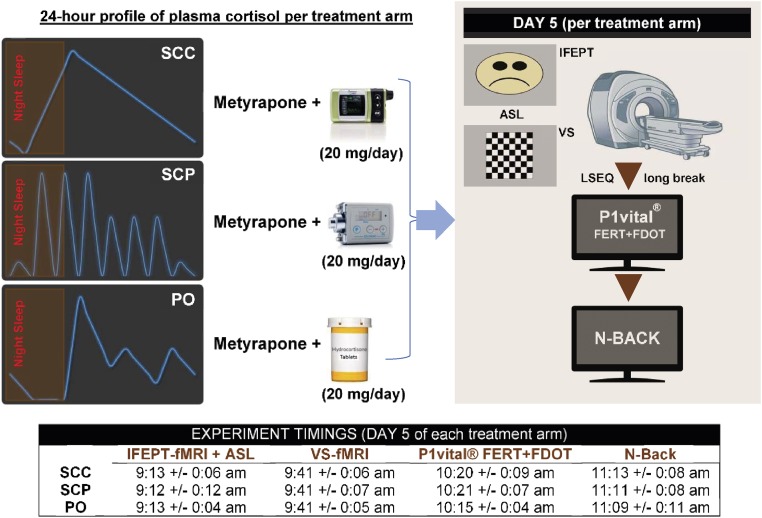
Fig. 1.
Study design. Each healthy male volunteer took part in three 5-d, randomized-order, block and replace studies. In each arm of the study, endogenous hydrocortisone was suppressed with metyrapone, and hydrocortisone was replaced at a dose of 20 mg/d via (i) s.c. with a normal circadian rhythm provided by continuous s.c. infusion with an Animas Vibe pump (SCC), (ii) pulsatile s.c. infusion providing both circadian and ultradian rhythms with a Canè CRONO P pump (SCP), or (iii) oral treatment (PO), using a three times daily regimen, resulting in just three pulses during the day and a prolonged low level at night. During each pharmacologic intervention, participants were given hydrocortisone/placebo tablets and were connected to one of the pumps (infusing placebo/hydrocortisone). Between pharmacologic interventions, there was a washout period of at least 2 wk. In the morning of the fifth day of each pharmacologic intervention, participants were attending the clinical facility and undergoing time-controlled functional (fMRI) and perfusion (ASL) magnetic resonance imaging experiments, completing an LSEQ and engaging with various computerized behavioral and cognitive tests. VS, visual stimulation task (flashing checkerboard). The mean timing (and the corresponding SD) per outcome measure per group is displayed.