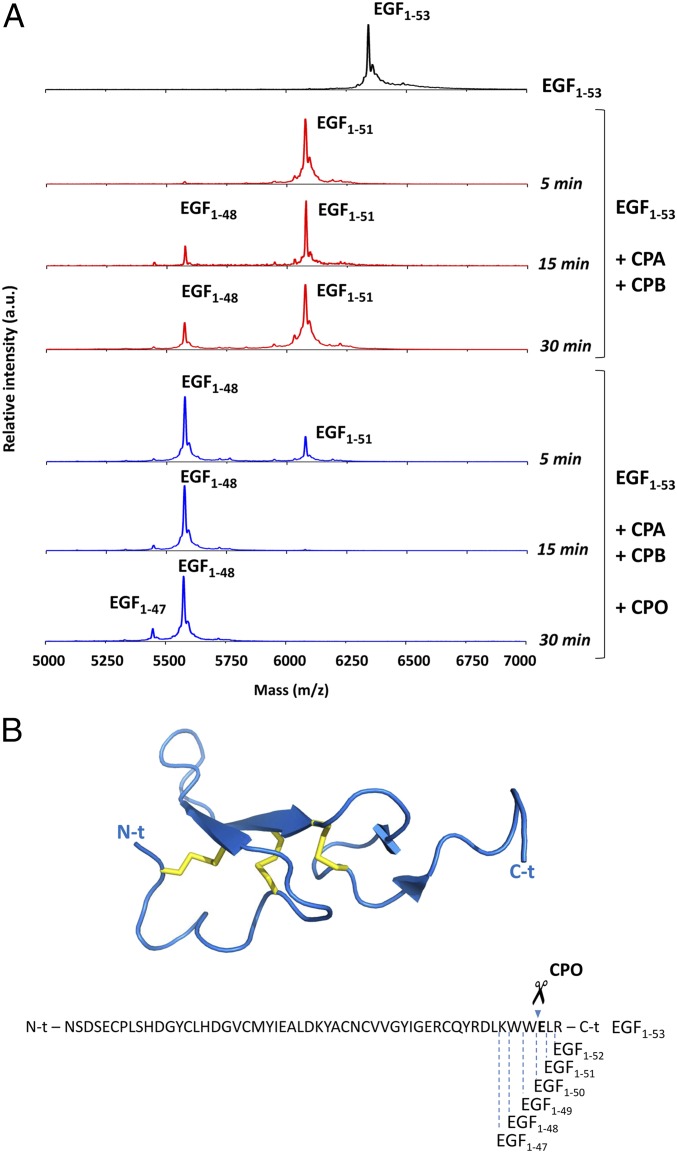
Fig. 5.
Substrate specificity of hCPOΔC in the C-t proteolytic cleavage of synthetic peptides and hEGF. (A) Representative MALDI-TOF MS spectra of full-length recombinant human EGF (EGF1–53) incubated for different times at 37 °C with 30 nM bCPA and 50 nM pCPB in the absence (red spectra) or in the presence (blue spectra) of 30 nM hCPOΔC. (B) Ribbon representation and amino acid sequence of human EGF. The C-t peptide bonds cleaved by the proteolytic action of bCPA, pCPB, and hCPOΔC are indicated below the sequence. The peptide bond that is mainly cleaved by hCPOΔC is indicated above the sequence with scissors. Note that the full-length recombinant human EGF used in the experiment contains the EGF1−53 sequence plus an additional N-t Met residue. Accordingly, the monoisotopic molecular masses are: EGF1−53 = 6,348.77 Da; EGF1−51 = 6,078.85 Da; EGF1−48 = 5,577.32 Da; EGF1−47 = 5,449.15 Da. Atomic coordinates of hEGF (PDB ID code 1IVO) were obtained from the Protein Data Bank (www.rcsb.org).