Abstract
Chuangxinmycin is an antibiotic isolated from Actinoplanes tsinanensis CPCC 200056 in the 1970s with a novel indole-dihydrothiopyran heterocyclic skeleton. Chuangxinmycin showed in vitro antibacterial activity and in vivo efficacy in mouse infection models as well as preliminary clinical trials. But the biosynthetic pathway of chuangxinmycin has been obscure since its discovery. Herein, we report the identification of a stretch of DNA from the genome of A. tsinanensis CPCC 200056 that encodes genes for biosynthesis of chuangxinmycin by bioinformatics analysis. The designated cxn cluster was then confirmed to be responsible for chuangxinmycin biosynthesis by direct cloning and heterologous expressing in Streptomyces coelicolor M1146. The cytochrome P450 CxnD was verified to be involved in the dihydrothiopyran ring closure reaction by the identification of seco-chuangxinmycin in S. coelicolor M1146 harboring the cxn gene cluster with an inactivated cxnD. Based on these results, a plausible biosynthetic pathway for chuangxinmycin biosynthesis was proposed, by hijacking the primary sulfur transfer system for sulfur incorporation. The identification of the biosynthetic gene cluster of chuangxinmycin paves the way for elucidating the detail biochemical machinery for chuangxinmycin biosynthesis, and provides the basis for the generation of novel chuangxinmycin derivatives by means of combinatorial biosynthesis and synthetic biology.
KEY WORDS: Chuangxinmycin, Actinoplanes tsinanensis, Biosynthesis gene cluster, Heterologous expression, Cytochrome P450, Seco-chuangxinmycin, C–S bond formation, Sulfur incorporation
Graphical abstract
In this study, the biosynthetic gene cluster of chuangxinmycin (1) was firstly identified, cloned and heterologously expressed and a plausible pathway for its biosynthesis was proposed: L-tryptophan was converted to seco-chuangxinmycin (3) through a series of biochemical reaction processes and by hijacking the sulfur transfer system from primary metabolism using the cluster-situated sulfur carrier protein CxnE. Then the second C–S bond formation was achieved by the cytochrome P450 CxnD.
1. Introduction
Chuangxinmycin (1, Fig. 1), discovered in the 1970s by scientists from Institute of Antibiotics (now Institute of Medicinal Biotechnology), Chinese Academy of Medical Sciences, is a secondary metabolite containing a novel indole-dihydrothiopyran heterocyclic skeleton isolated from Actinoplanes tsinanensis CPCC 2000561. It showed in vitro antibacterial activity against both Gram-negative and Gram-positive bacteria, and in vivo efficacy in mouse infection models against Escherichia coli and Shigella dysenteriae, as well as in preliminary clinical trials for septicaemia, urinary and biliary infections caused by E. coli1. Although chuangxinmycin did not come into clinical use, its unique antibacterial mechanism as a bacterial tryptophanyl tRNA synthetase (TrpRS) inhibitor2 and the unusual heterocyclic skeleton with a sulfur have attracted the interests of pharmacologists and medicinal chemists, especially recently when the antibiotic-resistant bacteria such as ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) become a serious emerging problem.
Figure 1.
Chemical structures of chuangxinmycin (1), demethyl-chuangxinmycin (2) and seco-chuangxinmycin (3).
Since the successful total organic synthesis of racemic chuangxinmycin in 19763, many derivatives of chuangxinmycin were obtained, a few of which showed improved anti-microbial activities4, 5, 6, 7, 8. However, biogenetic origins and synthetic mechanisms of chuangxinmycin have remained largely obscure for over 40 years. The preliminary investigation into the biosynthetic pathway for chuangxinmycin has started since its discovery. For example, Cao et al.9 identified a methyltransferase activity with specificity for 3-indolylpyruvate from A. tsinanensis, which might have played a role in chuangxinmycin biosynthesis. Xu et al.10 proposed that the sulfur atom in chuangxinmycin might come from cysteine by feeding [35S]-cystine during the fermentation of A. tsinanensis. Zhou et al.11 suggested vitamin B12 is indispensable for the biosynthesis of chuangxinmycin. Since then, no significant progress has been achieved on chuangxinmycin biosynthesis.
Structurally, chuangxinmycin bears some resemblance to indolmycin, another known inhibitor of TrpRS12. It is also somehow similar to l-tryptophan, the substrate of TrpRS. Therefore, tryptophan is generally regarded as the precursor for chuangxinmycin biosynthesis. On the other hand, sulfur is essential and ubiquitous in living systems, thus the incorporation of sulfur atom is the most remarkably fascinating biosynthetic feature. Although sulfur atom appears in many primary and some secondary microbial metabolites, knowledge about the intriguing enzymatic machinery driving the incorporation of sulfur atom into microbial secondary metabolites remains limited. Recently, Hungwen Liu's group13 disclosed a strategy of secondary metabolite biosynthesis by hijacking the primary sulfur-delivery system including sulfur carrier protein (SCP), SCP-activating enzyme, etc. Wen Liu's group14 established the metabolic coupling of two small-molecule thiols, mycothiol and ergothioneine, in the biosynthesis of lincomycin A. These findings provide valuable clues for analyzing the biosynthesis pathway of dihydrothiopyran ring which will facilitate the identification of biosynthetic gene cluster of chuangxinmycin in the genome of A. tsinanensis CPCC 200056.
The advent of next-generation sequencing technologies has revolutionized the search for novel antibiotic biosynthetic gene cluster which is of great importance in proposing a plausible biosynthetic pathway in its producing microorganism. In this work, we report the successful mapping of putative chuangxinmycin biosynthetic gene cluster (cxn) in the genome of A. tsinanensis CPCC 200056 by searching adjacent genes encoding: (1) TrpRS, which is supposed to be a self-resistant mechanism for chuangxinmycin producer; (2) aminotransferase and methyltransferase, which are responsible for the transformation of l-tryptophan; and (3) protein members of primary sulfur transfer system or low-molecular-mass thiol biotransformations, which might be involved in the incorporation of sulfur atom. We describe the identification and characterization of the cxn biosynthesis gene cluster and propose a rational and convincing chuangxinmycin biosynthetic pathway in A. tsinanensis CPCC 200056.
2. Materials and methods
2.1. Strains, plasmids and growth conditions
All the strains and plasmids used in this study are listed in Table 11, 15, 16, 17, 18, 19, 20, 21. The chuangxinmycin-producing strain A. tsinanensis CPCC 200056, obtained from China Pharmaceutical Culture Collection, was grown at 28 °C on solid ISP222 medium for sporulation and fermentation. The medium 65 plates (4 g/L of glucose, 4 g/L of yeast extract, 10 g/L of malt extract, 2 g/L of CaCO3, 12 g/L of agar, pH 7.2)23 were used for conjugation between A. tsinanensis and E. coli. The heterologous expression host strain Streptomyces coelicolor M114615 and its derivatives were cultured at 28 °C on Mannitol soya flour (MS) agar24, which was also used for conjugation between Streptomyces and E. coli. Liquid phage medium25 were used for isolation of genomic DNA. E. coli DH5α17 was used as a host for general cloning experiments. E. coli ET12567/pUZ800218 were used for conjugal transfer according to the established protocol. All of the E. coli strains were incubated in Luria–Bertani medium (LB) at 37 °C. Yeast YPAD medium containing 1% yeast extract, 2% peptone and 2% dextrose supplemented with 0.01% adenine hemisulfate was used to grow Saccharomyces cerevisiae VL6-48 (MATα, his3-Δ200, trp1-Δ1, ura3-52, lys2, ade2-101, met14, psi+cir0)16 at 30 °C, which was used as the host for DNA assembler. Synthetic tryptophan dropout agar (SD-Trp agar, purchased from Clontech, California, USA) was used to select yeast transformants containing the assembled biochemical gene clusters of interest. Apramycin (Am, 50 μg/mL), kanamycin (Km, 50 μg/mL), chloramphenicol (Cm, 30 μg/mL), hygromycin B (Hyg, 200 μg/mL) and nalidixic acid (25 μg/mL) were used for selection of E. coli and Streptomyces recombinant strains.
Table 1.
Strains and plasmids used in this study.
| Strains/plasmid | Relevant characteristic | Ref. |
|---|---|---|
| Strain | ||
| A. tsinanensis CPCC 200056 | Wild-type strain (chuangxinmycin-producing strain) | 1 |
| S. coelicolor M1146 | The heterologous expression host strain, Δact, Δred, Δcpk, Δcda | 15 |
| S. coelicolor M1146/pCAP-CM(ΔcxnD) | A copy of cxn biosynthetic gene cluster* (cxnD inactivated) was integrated on the chromosome of S. coelicolor M1146, Amr | This study |
| S. coelicolor M1146/pCAP-CM | A copy of cxn biosynthetic gene cluster* (cxnD inactivated) and a complement coding region of cxnD were integrated on the chromosome of S. coelicolor M1146, Amr, Hygr | This study |
| S. coelicolor M1146/pCAP-CM(ΔcxnA~F) | The majority of genes (cxnA~F) in cxn cluster were deleted in S. coelicolor M1146, Amr | This study |
| S. cerevisiae VL6-48 | The homologous recombination host for DNA assembly, MATα, his3-Δ200, trp1-Δ1, ura3-52, lys2, ade2-101, met14, psi+cir0 | 16 |
| E. coli | ||
| DH5α | General cloning host | 17 |
| ET12567/pUZ8002 | Donor strain for intergeneric conjugation between E. coli and S. coelicolor and A. tsinanensis, Cmr, Kmr | 18 |
| Plasmid | ||
| pCAP01 | Gene cluster capture vector; ARSH4/CEN6, pUC ori, aph(3)II, ϕC31 int/attP, oriT (RP4) | 16 |
| pCAP-CM-LR | The cxn gene cluster specific capture vector with two homology arms corresponding to flanking regions of the target gene cluster into pCAP01, Kmr | This study |
| pCAP01-CM | pCAP01 carrying the entire cxn biosynthetic gene cluster* (cxnD inactivated), Kmr | This study |
| pCAP-CM(ΔcxnD) | A derivative of pCAP01-CM, Amr | This study |
| pCAP-CM(ΔcxnA~F) | pCAP-CM(ΔcxnD) derivative plasmid with deletion of the majority of cxn genes (cxnA~F), Amr | This study |
| pIJ10500 | Streptomyces integrative vector containing ϕBT1 integrase gene (int) and its attachment site (attP), Hygr | 19 |
| pIJ-CxnD | pIJ10500 derivative plasmid containing complete coding region of cxnD downstream of ermE*p, Hygr | This study |
| pSET152 | Streptomyces integrative vector consisting of the ϕC31 integrase gene (int) and its attachment site (attP), Amr | 20 |
| pL646 | pSET152 derivative containing the constitutive promoter ermE*p, Amr | 21 |
| pL-CxnR | pL646 derivative plasmid containing 942 bp complete coding region of CxnR, Amr | This study |
Amr, apramycin resistance; Kmr, kanamycin resistance; Cmr, chloramphenicol resistance; Hygr, hygromycin B resistance; S. coelicolor, Streptomyces coelicolor; A. tsinanensis, Actinoplanes tsinanensis; S. cerevisiae, Saccharomyces cerevisiae; E. coli, Escherichia coli.
2.2. Genomic DNA extraction and genome sequencing
Total DNAs were extracted from A. tsinanensis CPCC 200056 grown in liquid phage medium at 28 °C for 48 h. Mycelium was harvested and washed with STE buffer (0.3 mol/L sucrose, 25 mmol/L Tris–HCl, 10 mmol/L EDTA, pH 8.0), then was lysed by STE buffer plus 5 mg/mL lysozyme and 1% SDS. Phenol-chloroform solution was used to remove proteins. The total DNAs was digested with DNase-free RNase A to remove RNAs, purified by isopropanol precipitation. Genomic DNA was sequenced with Pacific Biosciences (PacBio) RSII SMRT technology and Illumina Hiseq. 4000 platform (commissioned to Beijing Genomics Institute, Shenzhen, China). The genome was directly assembled by SMRT Analysis v2.3.026 based on the subreads obtained from Pacbio RSII platform. SOAPsnp27, SOAPindel28 and GATK29 were used to correct the errors found in the assembled sequence based on the data obtained from Illumina Hiseq. 4000 platform. Putative protein-coding sequences (CDSs) were predicted using Glimmer 3.030. Gene functional annotation was performed using BLASTP with KEGG, COG, Swiss-Port, TrEMBL, NR and GO databases.
2.3. Intergeneric conjugation between E. coli and A. tsinanensis
The vector pL64621, a pSET15220-derived expression plasmid containing a ϕC31 attP-int locus, was used for intergeneric conjugation between E. coli and A. tsinanensis (Table 1). A 942 bp DNA fragment containing the complete cxnR- coding region was amplified using pL-CxnR-F and pL-CxnR-R as PCR primers (Table S1 in Supplementary information), and cloned into the NdeI and BamHI sites of pL646, under the control of a strong constitutive promoter ermE*p, to give pL-CxnR. Then it was transferred into methylase-negative strain E. coli ET12567/pUZ8002 which was used as the E. coli donor strain. The A. tsinanensis recipient strain was cultivated in 100 mL of CRM medium24 in a 500-mL baffled Erlenmeyer flask which was inoculated with one cultured square (about 1 cm×1 cm in size). The incubation was carried out at 28 °C for 5 days on a rotary shaker (Zhicheng, Shanghai, China) at 200 rpm. The culture was diluted 1 to 10 and further incubated in 50 mL of tryptic soy broth (TSB) medium (BD, Franklin Lakes, USA) for 16 h under the same conditions. Then, the mycelium was homogenized and diluted 1 to 5 (10 mL TSB plus 2.5 mL of mycelium), and incubation was carried out for from 1 h to a maximum of 5 h under the conditions described above. For the conjugation approach, the cells were pelleted by centrifugation (5000×g, 10 min) and resuspended in 2 mL of TSB. A total of 200 μL of A. tsinanensis cells (approximately 108 cells/mL) and 200 μL of the E. coli donor strain (a washed overnight culture; approximately 108 cells/mL) were mixed and then spread onto medium 65 plates containing 20 mmol/L MgCl2. After 16–18 h, the plates were overlaid with 3 mL of H2O containing nalidixic acid and apramycin (A. tsinanensis is intrinsically resistant to apramycin). After 5–7 days, the transconjugants were picked onto ISP2 agar containing apramycin at 28 °C for 7 days and then inoculated into liquid phage medium for isolation of the genomic DNA for PCR verification using pSET152 and attB_Streptomyces as primers (Table S1).
2.4. Chuangxinmycin biosynthetic gene cluster assembly using DNA assembler
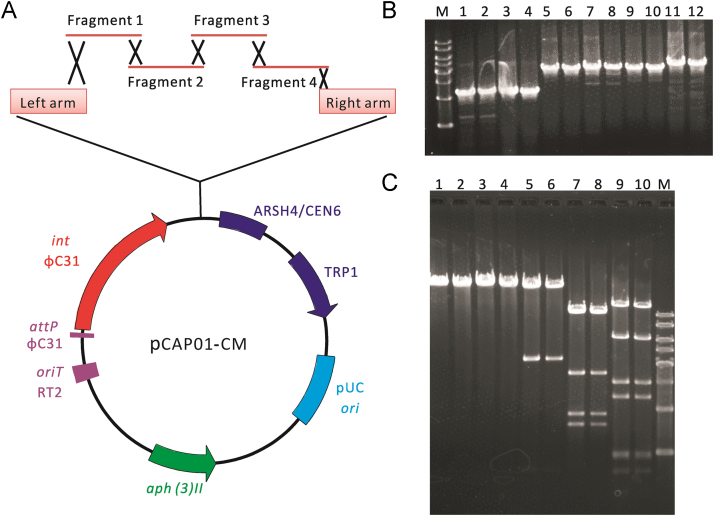
The capture vector pCAP0116 has three elements which were the yeast element consisting of ARSH4/CEN6 (replication origin) and TRP1 auxotrophic marker, the E. coli element containing the pUC ori from SuperCos1 (Stratagene, La Jolla, USA) and the Streptomyces element consisting of the ϕC31 integrase gene (int) and its attachment site (attP), origin of DNA transfer (oriT), and kanamycin resistance gene (aph(3)II), respectively. The target cluster-specific capture vector was constructed by introducing into the vector two short homology arms (~2 kb) corresponding to flanking regions of the target gene cluster. Two arms and gene cluster fragments were amplified from the genomic DNA of A. tsinanensis using primers whose sequences are listed in Table S1. The two PCR amplified capture arms were introduced into pCAP01 by restriction/ligation, yielding the target cluster-specific capture vector pCAP-CM-LR. Then the “circular” construct was digested with EcoRI, and dephosphorized with calf intestine alkaline phosphatase, generating linear capture vector flanked by 2 kb capture arms at both ends (Fig. 2). To ensure high efficiency of yeast homologous recombination, each fragment was built to generate a 100–800 bp overlap region with adjacent fragments. 500–600 ng of each individual cassette/fragment (~4.0 kb) and 2 μg linear capture vector were mixed and precipitated with ethanol. The resulting DNA pellet was air-dried and resuspended in 4 μL of Milli-Q double deionized water. The concentrated mixture of DNA was electroporated into S. cerevisiae using the protocol reported elsewhere31. Desired transformants were selected on synthetic tryptophan dropout agar (SD-Trp agar) at 30 °C for 2–3 days and firstly identified by colony PCR. The correct clones with the target cluster were further confirmed by restriction analysis. Cells were spheroplasted using Zymolyase-20T (MP Biomedicals, Irvine, USA) at 37 °C for 0.5–1 h and harvested by centrifugation at 2000×g for 10 min. Then the plasmid DNA was purified following the protocols of HiPure BAC DNA Kits (Magen, Guangzhou, China) and introduced into E. coli DH5α by chemical transformation or electroporation. The plasmids were then purified from Kmr E. coli clones, and confirmed by restriction mapping/analysis with SpeI, NdeI, EcoRI, KpnI and XhoI. The yielded construct was designated as pCAP01-CM.
Figure 2.
Organization of the chuangxinmycin biosynthetic gene cluster (cxn) of A. tsinanensis. Left arm and right arm represent the capture arms in the direct cloning of the cluster. “Deleted” represents the deletion fragment in pCAP-CM(ΔcxnA~F).
2.5. Heterologous expression in S. coelicolor M1146
To facilitate the following conjugation, the kanamycin resistance gene aph(3)II in pCAP01-CM was replaced by apramycin resistance gene aac(3)IV using PCR-targeting. Then the new plasmid was sequenced and a point mutation was present (the GAA codon of Glu149 in CxnD was changed to termination codon TAA) which led to early termination of CxnD translation, so it was designated as pCAP-CM(ΔcxnD). When pCAP-CM(ΔcxnD) was digested by EcoRI, the 23 kb DNA fragment (with the deletion of ats4178~4183) was extracted from agarose gel and ligated by itself, produced the plasmid pCAP-CM(ΔcxnA~F) deleting the majority of biosynthetic genes. The pCAP-CM(ΔcxnD) and pCAP-CM(ΔcxnA~F) were introduced into E. coli ET12567/pUZ8002 and transferred to S. coelicolor M1146 by intergeneric conjugation according to a standard protocol24. Amr exconjugants were selected on MS agar containing nalidixic acid and apramycin at 28 °C and then inoculated into liquid phage medium for isolation of the genomic DNA. The correct exconjugants were verified by PCR (Fig. S2) and designated as S. coelicolor M1146/pCAP-CM(ΔcxnD) and M1146/pCAP-CM(ΔcxnA~F), respectively. The early termination of CxnD may cause functional inactivation in S. coelicolor M1146/pCAP-CM(ΔcxnD), so the complete coding region of cxnD was introduced into S. coelicolor M1146/pCAP-CM(ΔcxnD) for functional complementation. The strong constitutive promoter ermE*p and coding region of cxnD were cloned to vector pIJ1050019 by Gibson assembly kit (NEB, Ipswich, USA), resulting the plasmid pIJ-CxnD. Then it was conjugated into S. coelicolor M1146/pCAP-CM(ΔcxnD) to get heterologous expression strain S. coelicolor M1146/pCAP-CM.
2.6. Production and analysis of chuangxinmycin and its derivatives
A. tsinanensis CPCC 200056, S. coelicolor M1146 and their derivatives were fermented on ISP2 medium at 28 °C for 7 days. Every culture on ISP2 plate was cut into squares (about 1 cm × 1 cm in size), and extracted once with 2-fold ethyl acetate overnight. The ethyl acetate was concentrated to the same volume (250–500 μL). The point injection volume during the silica gel TLC analysis was 5 μL. The developing solvent system includes ethyl acetate/n-hexane/dichloromethane/glacial acetic acid (9:7:6:0.2, v/v/v/v), observed at 254 and 365 nm respectively. The final ethyl acetate extract was filtered with organic membrane filter of 0.22 μm and the filtrate was analyzed by HPLC. The HPLC conditions were as follows: XSelect CSHTM C18 column (150 mm × 4.6 mm, 5 μm, Waters, Ireland), or Eclipse Plus C18 column (150 mm × 4.6 mm, 5 μm, Agilent, USA), or CAPCELL PAK ADME column (250 mm × 4.6mm, 5 μm, SHISEIDO, Japan), mobile phase MeCN–H2O (water phase containing 0.1% glacial acetic acid), 15%–100%, 30 min, flow rate of 1.0 mL/min, detection wavelength of 254 nm and column temperature of 25 °C. LC–MS analysis was monitored by HPLC as described above (except the flow rate decreased to 0.8 mL/min) coupled to an ESI mass spectrometer (1290 (LC)-1956 single quadrupole MS (Agilent, USA) or 1100 (LC)-6300 MSD Trap MS (Agilent, USA)). Electrospray ionization (negative ionization) in Ultra Scan mode with a capillary voltage of 3.5 kV and a heated capillary temperature of 325 °C was used for LC–MS analysis. The compound 3 (Fig. 1) was prepared by HPLC chromatography as described above from the ISP2 culture of S. coelicolor M1146/pCAP-CM(ΔcxnD). The HR-ESI-MS and HR-ESI-MS/MS analysis of the standard compound 1 (chemically synthesized3) and 3 was achieved by a QSTARTM Elite LC–MS/MS system (Applied Biosystems/MSD Sciex, Singapore).
3. Results and discussion
3.1. Bioinformatic identification of the chuangxinmycin biosynthesis gene cluster
The genome sequence of A. tsinanensis CPCC 200056 was obtained using a third generation sequencing platform Pacbio RSII, together with a second generation sequencing platform Illumina Hiseq. 4000, and yielded approximately 7.7 Mb of contiguous sequence plus one 13.5 kb circular plasmid with a GC content of 70.3% and 69.0%, respectively. The genome of CPCC 200056 totally contains 7060 protein coding genes, accounting for 87.03% of the genome.
Chuangxinmycin is a potent and selective inhibitor of bacterial tryptophanyl tRNA synthetase (TrpRS)2, thus we speculated that a tryptophanyl-tRNA synthetase gene (trpRS) might colocalize with the chuangxinmycin biosynthetic genes, as resistant and biosynthetic genes for a specific secondary metabolite are usually clustered in bacteria. Manual BLASTP searching of the A. tsinanensis CPCC 200056 genomic data uncovered two putative trpRS genes throughout the genome, and ats4186 drew our attention. Sequence analysis of its neighboring genes showed the presence of an aminotransferase gene (ats4179) with significant similarity with the tryptophan aminotransferase (ind8 and orf2651) involved in the indolmycin biosynthetic pathway32. The biosynthetic gene cluster of indolmycin, another known inhibitor of TrpRS, has recently been identified, suggesting the involvement of a tryptophan aminotransferase (ind8 and orf2651) as the first step in its biosynthetic pathway to convert tryptophan to indolepyruvate. More intriguing, an SCP gene (ats4181) was present in the neighborhood, suggesting its possible involvement in the sulfur incorporation into the chuangxinmycin molecule. Thus, we hypothesized that this gene cluster, named the cxn cluster, is potentially involved in the chuangxinmycin biosynthetic pathway.
The organization of cxn cluster is shown in Fig. 2 and the proposed function of each ORF is given in Table 2. Sequence analysis of this gene cluster predicts that cxnA (ats4178) encodes a cobalamin (vitamin B12)-dependent radical S-adenosylmethionine (SAM) methyltransferase. cxnB (ats4179) encodes a pyridoxal 5′-phosphate (PLP)-dependent aminotransferase. cxnC (ats4180) is homologous to dehydropantoate reductase. cxnD (ats4182) encodes a cytochrome P450. cxnE (ats4181) encodes an SCP gene. cxnF (ats4183) encodes a protein with a MPN domain, belonging to JAMM (JAB1/MPN/Mov34) metalloprotease family33. cxnT (ats4184) encodes a transporter of major facilitator superfamily. cxnR (ats4185) encodes an LysR family transcriptional regulator and trpRS (ats4186) encodes a putative resistant gene. Genes flanking this putative cxn cluster were also analyzed. ats4177 encodes a hypothetic protein of unknown function. Ats4176 is homologous to a peptidoglycan-binding protein. ats4175 encodes an HTH_XRE family transcriptional regulator. ats4187 encodes a membrane protein, ats4188 encodes an ion-coupled transporter, and ats4190 encodes a protein of unknown function (Table 2).
Table 2.
Proposed chuangxinmycin biosynthetic gene cluster and gene functions.
| Gene | cxn name | Size (aa) | Protein homologue | Identity/Similarity (100%) | Proposed function |
|---|---|---|---|---|---|
| ats3059 | 264 | ThiG, thiazole synthase (WP_055570175.1), Streptomyces atriruber | 94/96 | Thiazole synthase | |
| ats4175 | 331 | Hypothetical protein (WP_047121450.1), Streptomyces leeuwenhoekii | 55/63 | HTH_XRE family transcriptional regulator | |
| ats4176 | 346 | Peptidoglycan-binding protein (WP_044378158.1), Streptomyces cyaneogriseus | 72/83 | Peptidoglycan-binding protein | |
| ats4177 | 240 | Hypothetical protein (WP_086718244.1), Streptomyces angustmyceticus | 81/85 | Associated to methylation | |
| ats4178 | cxnA | 623 | Maturation radical SAM protein 1 (WP_063754376.1 RiPP), Streptomyces sp. NRRL S-1813 | 93/96 | Radical SAM protein, methylation |
| ats4179 | cxnB | 362 | ThnJ, aminotransferase (AMR44310.1), Streptomyces sp. FXJ1.172 | 52/63 | Aminotransferase |
| ats4180 | cxnC | 318 | 2-Dehydropantoate 2-reductase (WP_030988196.1), Streptomyces sp. NRRL S-1813 | 87/90 | Reductase |
| ats4182 | cxnD | 404 | ThnC, P450 (AMR44303.1), Streptomyces sp. FXJ1.172 | 37/48 | Cytochrome P450 |
| ats4181 | cxnE | 100 | MoaD family protein (KRT67113.1), Candidatus Dadabacteria CSP1-2 | 43/71 | Sulfur-carrier protein |
| ats4183 | cxnF | 238 | ThnF, hypothetical protein (AMR44306.1), Streptomyces sp. FXJ1.172 | 44/61 | JAMM (JAB1/MPN/Mov34) metalloprotease |
| ats4184 | cxnT | 496 | MFS transporter (WP_051818417.1), Streptomyces sp. NRRL S-1813 | 86/90 | Transporter |
| ats4185 | cxnR | 313 | LysR family transcriptional regulator (AJK58337.1), Amycolatopsis lurida NRRL 2430 | 37/50 | Pathway-specific regulator |
| ats4186 | trpRS | 300 | Ind0, tryptophanyl-tRNA synthetase (AJT38681.1), Streptomyces griseus subsp. griseus | 73/83 | Tryptophan-tRNA synthetase |
| ats4187 | 846 | Membrane protein (ANZ15560.1), Streptomyces noursei ATCC 11455 | 39/52 | Membrane protein | |
| ats4188 | 548 | Sodium/proline symporter (WP_037838132.1), Streptomyces sp. NRRL S-337 | 68/81 | Transporter | |
| ats4190 | 79 | No homologous protein | Unknown | ||
| ats6133 | 392 | Adenylyltransferase/sulfurtransferase MoeZ (WP_067275253.1), Streptomyces jeddahensis | 98/98 | SCP-activating enzyme |
3.2. Overexpression of cluster-situated regulator CxnR
There is a possible pathway-specific regulator CxnR situated in the predicted cxn cluster. It is homologous to LysR family transcriptional regulator in Amycolatopsis lurida NRRL 2430 (37% identity) and FkbR1 (35% identity) which was a positive regulator for ascomycin production in S. hygroscopicus var. ascomyceticus ATCC 1489134. The effect of overexpression of the regulator CxnR in wild type strain on the production of chuangxinmycin was detected to determine whether the gene cluster was responsible for the biosynthesis of chuangxinmycin.
The overexpression plasmid pL-CxnR was introduced into the wild type strain A. tsinanensis CPCC 200056 by conjugation to give the overexpression strain 200056/pL-CxnR, with pSET152 transferred strain 200056/pSET152 as a control. Then these strains were cultured simultaneously on ISP2 agar with the same inoculum size and the fermentation product was extracted with ethyl acetate and analyzed by TLC and HPLC. The result of TLC displayed that the 1 production in 200056/pL-CxnR was significantly improved than that in 200056/pSET152 (Fig. S1 in Supplementary information). Furthermore, relative quantitative analysis was carried out by HPLC and showed that the production level of 1 in 200056/pL-CxnR was increased to 198% compared with that in 200056/pSET152 (Fig. 3). These results suggest that CxnR is the pathway-specific positive regulator for chuangxinmycin biosynthesis and support that the predicted cluster is responsible for the production of chuangxinmycin.
Figure 3.
Comparison of chuangxinmycin production between overexpression strain 200056/pL-CxnR and control strain 200056/pSET152. (A) The HPLC profile of representative conjugant of 200056/pL-CxnR and 200056/pSET152; (B) Quantitative comparison of 1 production (statistical results of 4 conjugants of 200056/pSET152 and 8 conjugants of 200056/pL-CxnR, respectively).
3.3. Direct cloning and heterologous expression of cxn cluster
To heterologously express the cxn cluster in S. coelicolor M1146, DNA assembler31 was used to directly clone the proposed cxn cluster by the in vivo homologous recombination mechanism in S. cerevisiae. Two capture arms (Fig. 2) were amplified and cloned into pCAP0116 to get the cxn cluster capture vector pCAP-CM-LR. Four fragments of the gene cluster were obtained using PCR and electroporated into S. cerevisiae together with linear pCAP-CM-LR, resulting in the construct pCAP01-CM carrying the entire gene cluster (ats4175~4190, Fig. 4A). The plasmid pCAP01-CM was isolated from S. cerevisiae, transferred into E. coli DH5α and then verified by PCR and restricted enzymatic analysis (Fig. 4B). To facilitate the following conjugation, the kanamycin resistance gene in pCAP01-CM was replaced with apramycin resistance gene by PCR-targeting35. However, sequencing of the assembled cxn cluster in this plasmid showed that there is a point mutation in cxnD which led to the early translation termination of CxnD (148 vs. 404 amino acids), thus this plasmid was named pCAP-CM(ΔcxnD). The further deletion plasmid pCAP-CM(ΔcxnA~F) with disruption of the majority of the cxn cluster was constructed by digestion of pCAP-CM(ΔcxnD) with EcoRI (there are 4 EcoRI sites situated within cxnA~F in this plasmid) and then re-ligation of the large fragment. These two plasmids were introduced into S. coelicolor M114615 by conjugation, resulting in two disruption strains S. coelicolor M1146/pCAP-CM(ΔcxnD) and S. coelicolor M1146/pCAP-CM(ΔcxnA~F). To fulfill the CxnD function in the disruption strain S. coelicolor M1146/pCAP-CM(ΔcxnD), the entire cxnD ORF was cloned into pIJ1050019, a plasmid containing a ϕBT1 attP-int locus. The resulting plasmid pIJ-CxnD, with cxnD under the control of promoter ermE*p, was transferred into S. coelicolor M1146/pCAP-CM(ΔcxnD) to give a complete cxn heterologous expression strain S. coelicolor M1146/pCAP-CM. The PCR primers used for verification of all the recombinant strains are shown in Table S1.
Figure 4.
Direct cloning of the cxn cluster by DNA assembler. (A) Schematic representation for the construction of pCAP01-CM; (B) PCR verification of pCAP01-CM isolated from E. coli DH5α. Lane M: 1 kb plus DNA ladder (10,000, 8000, 6000, 5000, 4000, 3000, 2000, 1500, 1000, 800, 500, 300 bp); Lane 1~2: amplification of the left arm (2265 bp); Lane 3~4: amplification of the right arm (2225 bp); Lane 5~6: amplification of the fragment 1 (4003 bp); Lane 7~8: amplification of the fragment 2 (4101 bp); Lane 9~10: amplification of the fragment 3 (4049 bp); Lane 11~12: amplification of the fragment 4 (4388 bp); (C) Restricted enzymatic analysis of pCAP01-CM isolated from E. coli DH5α. Lane 1~2: SpeI (28,242 bp); Lane 3~4: NdeI (28,242 bp); Lane 5~6: EcoRI (23,132, 4336 bp); Lane 7~8: KpnI (10,834, 10,483, 3340, 1884, 1601 bp); Lane 9~10: XhoI (12,553, 6319, 3031, 2415, 1002, 951, 714, 444, 399, 237, 141 bp); Lane M: 1 kb DNA ladder (10,000, 8000, 6000, 5000, 4000, 3000, 2000, 1000 bp).
The three recombinant strains S. coelicolor M1146/pCAP-CM, M1146/pCAP-CM(ΔcxnD) and M1146/pCAP-CM(ΔcxnA~F), together with control strain M1146/pSET152 and chuangxinmycin producing wild type strain A. tsinanensis CPCC 200056, were cultured on ISP2 agar plates at 28 °C for 7 days. The culture was extracted with ethyl acetate and concentrated, and then analyzed by liquid chromatograph—mass spectrometer (LC–MS). Compared with that of control strain M1146/pSET152, two new HPLC peaks appeared at 18.1 and 16.9 min in the products of strain M1146/pCAP-CM containing complete cxn cluster (Fig. 5), with the maximum absorption wavelength (λmax) at the expected 230 and 300 nm, which were the same as those of chuangxinmycin (1) and demethyl-chuangxinmycin (2, Fig. 1), previously characterized in the wild type strain A. tsinanensis CPCC 20005622. In the LC–MS analysis, the two peaks revealed molecular ions at m/z 232 and 218 ([M–H]—) respectively, identical to those of 1 and 2. In the disruption strain M1146/pCAP-CM(ΔcxnA~F), these two peaks were not detected. These results demonstrated that the predicted cxn cluster was responsible for the biosynthesis of chuangxinmycin.
Figure 5.
Heterologous expression of cxn cluster in S. coelicolor M1146. (A) HPLC analysis of different strains; (B) Molecular ion peaks of 1, 2 and 3 in A; (C) UV absorption spectra of 1, 2 and 3.
LC–MS analysis of extracts from the recombinant strain M1146/pCAP-CM(ΔcxnD) showed loss of 1 and 2 production, and the appearance of a new UV-absorbing peak (3) at a retention time of 18.8 min, displayed a molecular ion of m/z 234 [M–H]−, 2 Da more than that of 1. The UV absorption profile of 3 is similar to 1 but with a blue shift of 10–20 nm (λmax at 220 and 280 nm), suggesting the decreased electron density of the indole aromatic group in 3. Thus the compound 3 was deduced as seco-chuangxinmycin (3-(1H-indol-3-yl)-2-thiolbutanoic acid) with an opened dihydrothiopyran ring compared with 1, as a biosynthetic intermediate in 1 biosynthesis. Further investigation of this metabolite using LC–ESI-HR-MS identified its m/z as 234.0601 for [M–H]− (molecular formula deduced as C12H12NO2S, Fig. S4, while Fig. S3 is for 1), corresponding to that of seco-chuangxinmycin (calculated for 234.0609 for M–1). Additional MS/MS analysis of 3 (Fig. 6B) revealed its fragmentation pattern to be analogous to that of 1 (Fig. 6A) to some extent. The fragment ions of m/z 173.9387 and 172.0233 in 3 and 1, respectively, were possibly derived by lost the carboxyl group and 3-methyl group of 3 and 1, respectively. The production of 3 in the recombinant strain M1146/pCAP-CM(ΔcxnD) verified that CxnD is involved in the second C–S bond formation (i.e., the closure of the dihydrothiopyran ring) in chuangxinmycin biosynthesis.
Figure 6.
HR-MS/MS analysis of 1 (A) and 3 (B).
3.4. Proposed pathway for chuangxinmycin biosynthesis
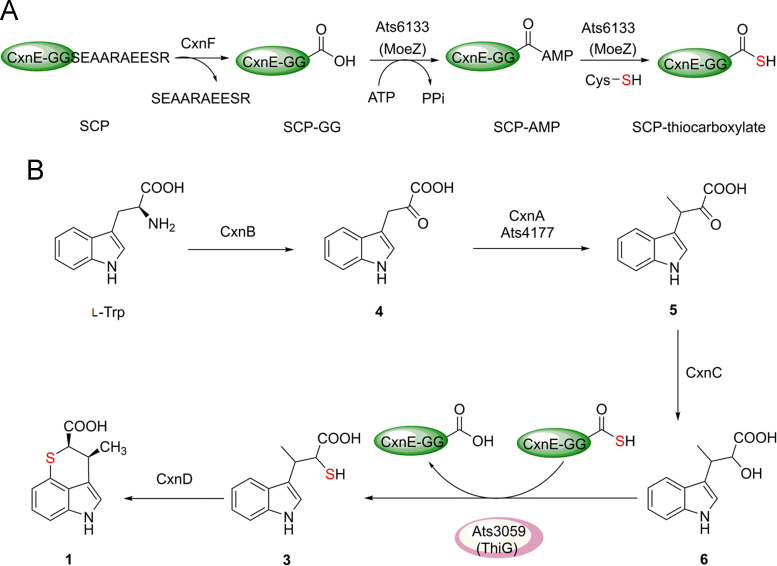
Chuangxinmycin bears a unique tricyclic indole-S-hetero scaffold as indolethiopyran that encompasses two C–S bonds. Thus, the most intriguing part of the biosynthesis is the mechanism of the incorporation of sulfur. Although the feeding of [35S]-cystine provided preliminary evidence that the sulfur atom is derived from cysteine10, the central metabolite of sulfur biochemistry in prokaryotic cells, the detailed mechanism of sulfur incorporation is still obscure. Fortunately, there is an SCP homologue (CxnE) in the cxn cluster we have identified, which strongly suggests that the first C–S bond is formed by hijacking the potential sulfur-transfer enzymes, including common SCP-activating enzymes, cysteine desulfurases, and rhodanese-like proteins from primary metabolism36. In the synthesis of thiamine, molybdopterin, thionucleosides, etc., the C–S bonds were formed using SCPs (ThiS, MoaD, etc.) activated by SCP-activating enzyme (ThiF, MoeB, etc.). Some secondary metabolites, such as thioquinolobactin37 and BE-7585A13, were reported to hijack the sulfur transfer systems from primary metabolism for incorporation of the sulfur into the secondary metabolite molecules. Recently, the mechanism of sulfur incorporation into the thiosugar of BE-7585A was elegantly described13. A ThiG (thiazole synthase for thiamin) homologue BexX is present in the BE-7585A biosynthetic cluster, and elsewhere in the genome a unique MoeZ (with a ThiF-like domain at N-terminus and a rhodanese homology domain at C-terminus) and several SCPs as well as cysteine desulfurases are present. The sulfur from L-cysteine charges the rhodanese domain of MoeZ mediated by a cysteine desulfurase, and then catalyzes thiolation of the adenylated SCP activated by ThiF-like domain of MoeZ. The resulting SCP-thiocarboxylate is the ready-to-use form to transfer the sulfur atom into the thiosugar of BE-7585A13.
The enzymatic machinery for sulfur incorporation in primary metabolism was also present in the genome of A. tsinanensis CPCC 200056. There are a unique ThiG homologous protein Ats3059 within the thiamin biosynthesis cluster and a unique MoeZ homologous protein Ats6133. Several cysteine desulfurases (Ats6445, Ats3108, Ats2758 and Ats3097) and SCP homologues (Ats3085, Ats3815, Ats4181, Ats4502 and Ats4708) are present, and among them, ats4181 is situated within the identified cxn cluster (Fig. 2). Thus, we envisaged that 1 utilized its own SCP (CxnE) and the primary sulfur transfer system, in a similar mechanism as used in the thiosugar of BE-7585A.
The C-terminus of the known SCPs such as ThiS and MoaD has a highly conserved -GG-COOH motif that functions as a flexible linker and activation site for thiolation. Comparison of CxnE with known SCPs showed that the GG motif is followed by a decapeptide SEAARAEESR in CxnE, i.e., it might present as a precursor of SCP. A close investigation of the ORFs in cxn clusters showed that CxnF contained an MPN domain and was supposed to be a member of JAMM metalloproteinase family. Furthermore, it has the conserved JAMM motif (EXnHS/THX7SX2D) (where X is any amino acid residue)38 for isopeptidase activity. In the biosynthesis of a sulfur-containing siderophore thioquinolobactin produced in Pseudomonas fluorescens33, the cluster situated SCP QbsE also has two more amino acids (CF) after the diglycine motif (GG). QbsD, a JAMM metalloproteinase, may digest CF of QbsE to expose GG as the new C-terminal for further adenylation and thiolation.
A summarized SCP-thiocarboxylate formation for 1 biosynthesis is proposed as in Fig. 7A. The C-terminal decapeptide SEAARAEESR of CxnE (an SCP) is first hydrolytically cleaved off by CxnF (a JAMM family metalloproteinase), which exposes GG as C-terminal dipeptides for the following activation. The adenylation of CxnE is achieved by the ThiF-like domain of MoeZ (Ats6133). The conserved cysteine residue in rhodanese domain of MoeZ is converted to a persulfide group through the action of a cysteine desulfurase using l-cysteine as the sulfur source. Then the adenylated CxnE is thiolated by persulfide group of rhodanese domain of MoeZ to produce SCP-thiocarboxylate.
Figure 7.
The proposed biosynthetic pathway of 1. (A) Activation of cxn cluster-situated SCP CxnE. (B) The proposed biosynthetic pathway of 1.
The chemical structure of 1 suggests the utilization of l-Trp as the starting material in a biosynthetic pathway, which is the case in another TrpRS inhibitor indolmycin32. Indolmycenic acid (6) has been reported as an intermediate in the biosynthesis of indolmycin32 and we predicted a similar mechanism in the biosynthesis of 1. The aminotransferase (Ind8 and Orf2651), C-methyltransferase (Ind1) and NADH-dependent dehydrogenase (Ind2) are responsible for the biosynthesis of 6. In the cxn cluster, CxnB shows high homology to a pyridoxal-5′-phophate (PLP)-dependent aminotransferase Ind8 and Orf2651 as well as ThnJ in thienodolin biosynthesis39, both responsible for the deamination of l-Trp to form indole-3-pyruvic acid (4). CxnA shows significant homology with C-methyltransferase of radical SAM superfamily enzyme GenK40, TsrM41 and ThnK42 in the biosynthesis of different secondary metabolites. CxnA is thus supposed to catalyze methyl transfer from SAM to the β carbon of 4 to produce 5. CxnC is a homologue of ketopantoate reductase PanE43 or ApbA44, catalyzing the NAD(P)H-dependent reduction of ketopantoate to pantoate in the pantothenate (vitamin B5) biosynthetic pathway. CxnC might be a functional equivalent of Ind2 to fulfill the reduction of ketone in 5 to hydroxyl in 6. Thus 6 is supposed to be an intermediate in 1 biosynthesis before the insertion of sulfur atom. Then the first C–S bond might be ready to be formed by the ThiG homologue (Ats3059) together with the CxnE-thiocarboxylate in a similar mechanism used by thiosugar of BE-7585A13.
Although CxnA shows low homology with C-methyltransferase Ind1, we speculate that they may catalyze the same reaction, as CxnA is a homologue of a vitamin B12-dependent SAM radical enzyme, catalyzing methylation of unreactive C or P atoms45. An indolepyruvate C-methyltransferase transforming indolepyruvic acid (4) to 5 was partially purified and characterized from A. tsinanensis CPCC 200056 in 19899, thus we speculated that the substrate of CxnA is 4. However, we could not exclude the possibility that demethyl-chuangxinmycin (2) or other intermediates might be the substrate of this methyltransferase, and the substrate specificity of CxnA warrant further investigations. Zhou et al.11 found that vitamin B12 could restore the production of 1 in 4 null mutants, and suggested it played an important role at late stage of 1 biosynthesis. This is consistent with the deduction that the CxnA is a vitamin B12-dependent C-methyltranferase, as well as that 2, probably produced by the null mutants, losing its antibacterial activity22. Interestingly, Ats4177 exhibits significant 3D structure homology with chaperone PqqD37 by the program HHpred46 although it shows no homology to any known protein by BLASTP. PqqD is a structurally conserved peptide chaperone protein that forms a ternary complex with PqqE, a radical SAM enzyme in the biosynthesis of pyrroloquinoline quinone. So Ats4177 might be associated with the C-methylation activity by forming protein complex with CxnA.
A cytochrome P450-mediated C–S bond formation has been elucidated in several microbial secondary metabolite biosynthesis, such as SgvP in griseoviridin biosynthesis47 and TlmF in five-membered thiolactone ring formation48. In thn cluster, a P450 (ThnC) was speculated to catalyze the second C–S bond formation in a similar manner in indolethiophene scaffold of thienodolin39. The P450 encoded by cxnD shows high homology with the above three P450s. The identification of the intermediate 3 in the S. coelicolor M1146 expressing cxnD-disrupted cxn cluster unambiguously verified that CxnD catalyzes the second C–S bond formation from 3. Our study provides a substantial basis for the elucidation of two different mechanisms in the formation of two C–S bonds in chuangxinmycin biosynthesis.
On the basis of these bioinformatic analysis and genetic experiments, a complete biosynthetic pathway for 1 can now be proposed (Fig. 7B): l-tryptophan is deaminated by CxnB (a tryptophan aminotransferase) to generate indole-3-pyruvic acid (4), which is then methylated by CxnA, a radical SAM superfamily protein as an indolepyruvate C-methyltransferase. The resulted 3-methyl-indolepyruvic acid (5) was reduced to indolmycenic acid (6) by CxnC (NAD(P)H-dependent reductase). The sulfur transfer system from primary metabolism is hijacked, together with the cluster-situated sulfur carrier protein CxnE as thiol group donor (processed by CxnF, activated and thiolated by MoeZ), to catalyze sulfur incorporation into 6 to form the first C–S bond in 3 (3-(1H-indol-3-yl)-2-thiolbutanoic acid). Finally, 3 is further cyclized by cytochrome P450 CxnD to form the second C–S bond in 1.
4. Conclusions
Bioinformatic analysis of the genome sequence of A. tsinanensis CPCC 200056 identified a contiguous stretch of DNA that contained some of the genes required for chuangxinmycin biosynthesis. We have unequivocally identified, cloned, genetically manipulated, and heterologously expressed the chuangxinmycin biosynthesis gene cluster (cxn) from A. tsinanensis. A plausible pathway was proposed mainly based on the in silico functional analysis of genes in cxn cluster, which provided new insights into chuangxinmycin biosynthesis by hijacking sulfur delivery system from primary metabolism. Furthermore, the involvement of the cytochrome P450 CxnD in the second C–S bond formation in chuangxinmycin biosynthesis was corroborated by the identification of an intermediate seco-chuangxinmycin in the cxnD-disrupted cxn heterologous expression strain. The feasibility of genetic manipulation of cxn cluster in S. coelicolor sets the basis for the future metabolic engineering and the direct production of novel chuangxinmycin derivatives.
Acknowledgments
We thank Bradley S. Moore (Scripps Institution of Oceanography, University of California, San Diego, USA) for kindly providing S. cerevisiae VL6-48 and plasmid pCAP01. We would like to thank Prof. Mervyn J. Bibb (John Innes Centre, Norwich, UK) for kindly providing Streptomyces coelicolor M1146 and pIJ10500. This work was supported by the National Natural Science Foundation of China (81621064, 81603006, 81402836 and 31170042), the National Mega-Project for Innovative Drugs (2015ZX09102007-016 and 2017ZX09101003-006-011) and the CAMS Initiative for Innovative Medicine (2016-I2M-3–012).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.apsb.2017.07.005.
Contributor Information
Linzhuan Wu, Email: wulinzhuan@imb.pumc.edu.cn.
Jiandong Jiang, Email: jiang.jdong@163.com.
Bin Hong, Email: binhong69@hotmail.com, hongbin@imb.pumc.edu.cn.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Chuangxinmycin Research Group Studies on a new antibiotic—chuangxinmycin. Sci Sin. 1977;20:106–112. [PubMed] [Google Scholar]
- 2.Qi T.Q., Liu X., Yang Y.F. Repression on the biosynthesis of enzymes involved in tryptophan synthetic pathway by chuangxinmycin. Acta Acad Med Sin. 1980;2:32–37. [PubMed] [Google Scholar]
- 3.Zhang Z.P., Xu X.D., Huang L.Z., Lin Y.Q., Li H.S., Yu Z.L. The total synthesis fo chuangxinmycin. Acta Chim Sin. 1976;34:133–142. [Google Scholar]
- 4.Guo X.L., Zhang Z.P. A new total synthesis of chuangxinmycin and the study of its stereoisomers. Acta Pharm Sin. 1987;22:671–678. [PubMed] [Google Scholar]
- 5.Yoshida T., Ito A., Ibusuki K., Murase M., Kotani E. Improved preparation of sulfur substituted 3-vinylpyrrole and its application to the syntheses of chuangxinmycin derivatives. Chem Pharm Bull. 2001;49:1198–1202. doi: 10.1248/cpb.49.1198. [DOI] [PubMed] [Google Scholar]
- 6.Brown M.J., Carter P.S., Fenwick A.S., Fosberry A.P., Hamprecht D.W., Hibbs M.J. The antimicrobial natural product chuangxinmycin and some synthetic analogues are potent and selective inhibitors of bacterial tryptophanyl tRNA synthetase. Bioorg Med Chem Lett. 2002;12:3171–3174. doi: 10.1016/s0960-894x(02)00604-2. [DOI] [PubMed] [Google Scholar]
- 7.Xu X.B., Liu J., Zhang J.J., Wang Y.W., Peng Y. Nickel-mediated inter-and intramolecular C–S coupling of thiols and thioacetates with aryl iodides at room temperature. Org Lett. 2013;15:550–553. doi: 10.1021/ol303366u. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z.Y., Zhu J.T., Jin J., Li Z.R. Synthesis and antibacterial activity evaluation of chuangxinmycin prodrugs. Chin Med Biotechnol. 2016;11:314–318. [Google Scholar]
- 9.Cao J., Qi T.Q. Study on indolepyruvic acid methyltransferase in chuangxinmycin-producing strain. Acta Microbiol Sin. 1989;29:63–67. [PubMed] [Google Scholar]
- 10.Xu J., Ma Y., Li Y. Studies on the biogenesis of sulfur in chuangxinmycin molecule. Acta Microbiol Sin. 1978;18:66–70. [Google Scholar]
- 11.Zhou X.Z., Lin L. Vitamin B12 plays an important role in biosynthesis of chuangxinmycin. Acta Acad Med Sin. 1984;6:109–111. [PubMed] [Google Scholar]
- 12.Werner R.G., Thorpe L.F., Reuter W., Nierhaus K.H. Indolmycin inhibits prokaryotic tryptophanyl-tRNA ligase. Eur J Biochem. 1976;68:1–3. doi: 10.1111/j.1432-1033.1976.tb10758.x. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki E., Zhang X., Sun H.G., Lu M.Y.J., Liu T.L., Ou A. Co-opting sulphur-carrier proteins from primary metabolic pathways for 2-thiosugar biosynthesis. Nature. 2014;510:427–431. doi: 10.1038/nature13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Q.F., Wang M., Xu D.X., Zhang Q.L., Liu W. Metabolic coupling of two small-molecule thiols programs the biosynthesis of lincomycin A. Nature. 2015;518:115–119. doi: 10.1038/nature14137. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Escribano J.P., Bibb M.J. Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb Biotechnol. 2011;4:207–215. doi: 10.1111/j.1751-7915.2010.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanaka K., Reynolds K.A., Kersten R.D., Ryan K.S., Gonzalez D.J., Nizet V. Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc Natl Acad Sci U S A. 2014;111:1957–1962. doi: 10.1073/pnas.1319584111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook J., Russell D.W. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor Laboratory Press; New York: 2001. [Google Scholar]
- 18.Paget M.S., Chamberlin L., Atrih A., Foster S.J., Buttner M.J. Evidence that the extracytoplasmic function sigma factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2) J Bacteriol. 1999;181:204–211. doi: 10.1128/jb.181.1.204-211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du D., Wang L., Tian Y., Liu H., Tan H., Niu G. Genome engineering and direct cloning of antibiotic gene clusters via phage var ϕBT1 integrase-mediated site-specific recombination in Streptomyces. Sci Rep. 2015;5:8740. doi: 10.1038/srep08740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bierman M., Logan R., O'Brien K., Seno E.T., Rao R.N., Schoner B.E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 21.Hong B., Phornphisutthimas S., Tilley E., Baumberg S., McDowall K.J. Streptomycin production by Streptomyces griseus can be modulated by a mechanism not associated with change in the adpA component of the A-factor cascade. Biotechnol Lett. 2007;29:57–64. doi: 10.1007/s10529-006-9216-2. [DOI] [PubMed] [Google Scholar]
- 22.Zuo L.J., Zhao W., Jiang Z.B., Jiang B.Y., Li S.F., Liu H.Y. Identification of 3-demethylchuangxinmycin from Actinoplanes tsinanensis CPCC 200056. Acta Pharm Sin. 2016;51:105–109. [PubMed] [Google Scholar]
- 23.Heinzelmann E., Berger S., Puk O., Reichenstein B., Wohlleben W., Schwartz D. A glutamate mutase is involved in the biosynthesis of the lipopeptide antibiotic friulimicin in Actinoplanes friuliensis. Antimicrob Agents Chemother. 2003;47:447–457. doi: 10.1128/AAC.47.2.447-457.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieser T., Bibb M.J., Buttner M.J., Chater K.F., Hopwood D.A. John Innes Foundation; Norwich, England: 2000. Practical streptomyces genetics. [Google Scholar]
- 25.Korn F., Weingartner B., Kutzner H.J. A study of twenty actinophages: morphology, serological relationship and host range. In: Freerksen E., Tarnok I., Thumin H., editors. Genetics of the actinomycetales. Gustav Fisher Verlag; New York: 1978. pp. 251–270. [Google Scholar]
- 26.Chin C.S., Alexander D.H., Marks P., Klammer A.A., Drake J., Heiner C. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 27.Li R.Q., Li Y.R., Fang X.D., Yang H.M., Wang J., Kristiansen K. SNP detection for massively parallel whole-genome resequencing. Genome Res. 2009;19:1124–1132. doi: 10.1101/gr.088013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S.T., Li R.Q., Li H., Lu J.L., Li Y.R., Bolund L. SOAPindel: efficient identification of indels from short paired reads. Genome Res. 2013;23:195–200. doi: 10.1101/gr.132480.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delcher A.L., Bratke K.A., Powers E.C., Salzberg S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics. 2007;23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao Z.Y., Luo Y.Z., Zhao H.M. DNA assembler method for construction of zeaxanthin-producing strains of Saccharomyces cerevisiae. Methods Mol Biol. 2012;898:251–262. doi: 10.1007/978-1-61779-918-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Y.L., Alkhalaf L.M., Ryan K.S. In vitro reconstitution of indolmycin biosynthesis reveals the molecular basis of oxazolinone assembly. Proc Natl Acad Sci U S A. 2015;112:2717–2722. doi: 10.1073/pnas.1419964112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godert A.M., Jin M., McLafferty F.W., Begley T.P. Biosynthesis of the thioquinolobactin siderophore: an interesting variation on sulfur transfer. J Bacteriol. 2007;189:2941–2944. doi: 10.1128/JB.01200-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song K.J., Wei L., Liu J., Wang J.H., Qi H.S., Wen J.P. Engineering of the LysR family transcriptional regulator FkbR1 and its target gene to improve ascomycin production. Appl Microbiol Biotechnol. 2017;101:4581–4592. doi: 10.1007/s00253-017-8242-4. [DOI] [PubMed] [Google Scholar]
- 35.Gust B., Challis G.L., Fowler K., Kieser T., Chater K.F. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A. 2003;100:1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller E.G. Trafficking in persulfides: delivering sulfur in biosynthetic pathways. Nat Chem Biol. 2006;2:185–194. doi: 10.1038/nchembio779. [DOI] [PubMed] [Google Scholar]
- 37.Evans R.L., Latham J.A., Xia Y.L., Klinman J.P., Wilmot C.M. Nuclear magnetic resonance structure and binding studies of PqqD, a chaperone required in the biosynthesis of the bacterial dehydrogenase cofactor pyrroloquinoline quinone. Biochemistry. 2017;56:2735–2746. doi: 10.1021/acs.biochem.7b00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birol M., Echalier A. Structure and function of MPN (Mpr1/Pad1 N-terminal) domain-containing proteins. Curr Protein Pept Sci. 2014;15:504–517. doi: 10.2174/1389203715666140221095109. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y.Y., Wang J.L., Yu S.Q., Wang F., Ma H.M., Yue C.W. Identifying the minimal enzymes for unusual carbon—sulfur bond formation in thienodolin biosynthesis. Chembiochem. 2016;17:799–803. doi: 10.1002/cbic.201500670. [DOI] [PubMed] [Google Scholar]
- 40.Kim H.J., McCarty R.M., Ogasawara Y., Liu Y.N., Mansoorabadi S.O., LeVieux J. GenK-catalyzed C-6' methylation in the biosynthesis of gentamicin: isolation and characterization of a cobalamin-dependent radical SAM enzyme. J Am Chem Soc. 2013;135:8093–8096. doi: 10.1021/ja312641f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierre S., Guillot A., Benjdia A., Sandström C., Langella P., Berteau O. Thiostrepton tryptophan methyltransferase expands the chemistry of radical SAM enzymes. Nat Chem Biol. 2012;8:957–959. doi: 10.1038/nchembio.1091. [DOI] [PubMed] [Google Scholar]
- 42.Marous D.R., Lloyd E.P., Buller A.R., Moshos K.A., Grove T.L., Blaszczyk A.J. Consecutive radical S-adenosylmethionine methylations form the ethyl side chain in thienamycin biosynthesis. Proc Natl Acad Sci U S A. 2015;112:10354–10358. doi: 10.1073/pnas.1508615112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matak-Vinković D., Vinković M., Saldanha S.A., Ashurst J.L., Von Delft F., Inoue T. Crystal structure of Escherichia coli ketopantoate reductase at 1.7 Å resolution and insight into the enzyme mechanism. Biochemistry. 2001;40:14493–14500. doi: 10.1021/bi011020w. [DOI] [PubMed] [Google Scholar]
- 44.Frodyma M.E., Downs D. ApbA, the ketopantoate reductase enzyme of Salmonella typhimurium is required for the synthesis of thiamine via the alternative pyrimidine biosynthetic pathway. J Biol Chem. 1998;273:5572–5576. doi: 10.1074/jbc.273.10.5572. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q., Van Der Donk W.A., Liu W. Radical-mediated enzymatic methylation: a tale of two SAMS. Acc Chem Res. 2012;45:555–564. doi: 10.1021/ar200202c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alva V., Nam S.Z., Söding J., Lupas A.N. The MPI bioinformatics Toolkit as an integrative platform for advanced protein sequence and structure analysis. Nucleic Acids Res. 2016;44:W410–W415. doi: 10.1093/nar/gkw348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie Y.C., Li Q.L., Song Y.X., Ma J.Y., Ju J.H. Involvement of SgvP in carbon—sulfur bond formation during griseoviridin biosynthesis. Chembiochem. 2014;15:1183–1189. doi: 10.1002/cbic.201400062. [DOI] [PubMed] [Google Scholar]
- 48.Tang X.Y., Li J., Moore B.S. Minimization of the thiolactomycin biosynthetic pathway reveals that the cytochrome P450 enzyme TlmF is required for five-membered thiolactone ring formation. Chembiochem. 2017;18:1072–1076. doi: 10.1002/cbic.201700090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material