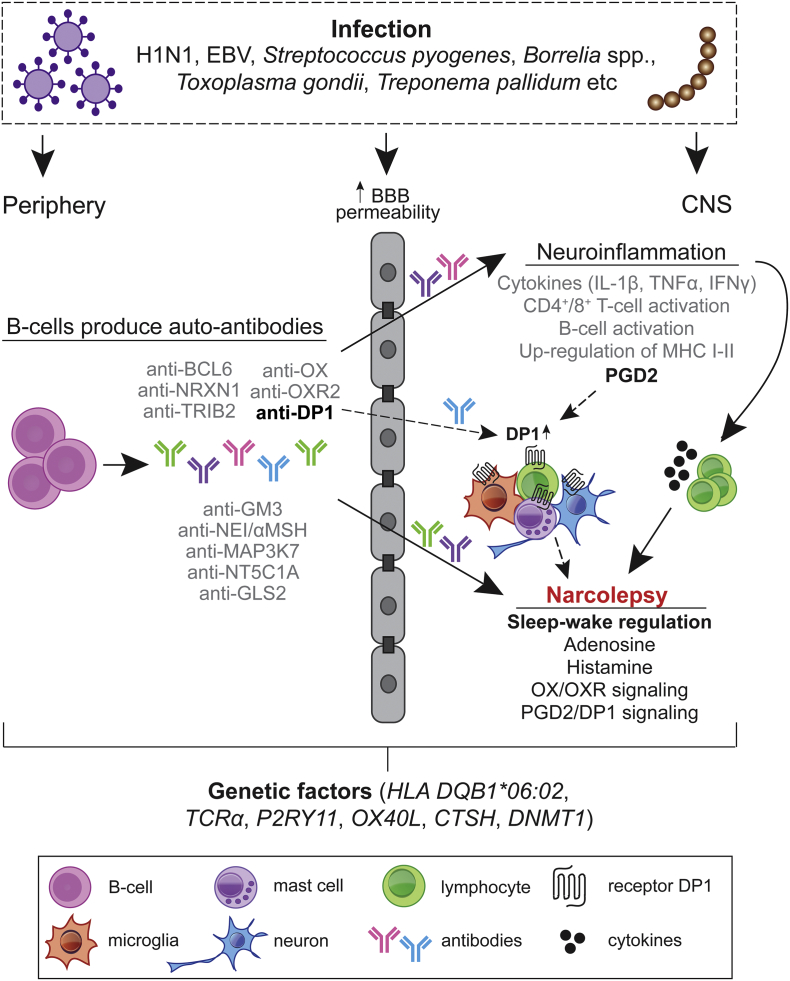
Fig. 7.
Hypothetical model for the aggravation of autoimmune response in Pdmx-associated and spontaneous NT1.
The immune response in NT1 is highly heterogenic with different pathways affected during the disease progression. We favor the idea that the lifelong risk for NT1 or for disease aggravation in pre-disposed individuals is increased following inflammatory triggers upon breaching of the blood–brain barrier (BBB) and with activation of preexisting auto-reactive antibodies (Ab) and cells reaching brain. Consequently, an immune response to A/H1N1 (and subsequent molecular mimicry) or a generalized stimulation of the immune system mediated by the Pdmx vaccine as AS03-adjuvanted vaccine can act as the inflammatory trigger (Morel et al., 2011; Carmona et al., 2010; Meyer et al., 2011). The inflammation triggers include i) infections (examples of pathogens are shown), ii) genetic factors, or iii) chronic inflammation (Kornum et al., 2011). The polyclonal Ab response from peripheral tissue may initiate disease by concentrating antigens in the brain to presentation-competent cells (Getahun et al., 2004). Recent data show further that peripherally produced human anti-CNS reactive antibodies are capable of opsonizing human CNS antigens (Kinzel et al., 2016). The entry of immune cells (T cells, B cells, macrophages, microglia and mast cells) cause neuroinflammation with the release of cytokines that damage neurons including HCRT+ neurons involved in sleep/wake regulation. Production of auto-reactive antibodies as a secondary response to cell death of HCRT-or other brain-resident cells can occur via antigen presenting cells. Prostaglandins are part of the inflammatory response in the brain acting via specific receptors. In particular, DP1 is produced by astrocytes, oligodendrocytes, neurons, microglia and meningeal cells (Liang et al., 2005; Mohri et al., 2007; Beuckmann et al., 2000). PGD2 signaling is known to prevent excessive inflammasome activation and may act as an anti-inflammatory pathway in the brain. Additionally, in brain residing mast cells, DP1 activity promotes maturation and histamine release (Taketomi et al., 2013). The latter is of particular interest given that histamine levels in the CSF of NT1-diseased are reduced (Nishino et al., 2009). Thus, our findings suggest that the anti-DP1 immune response, whether causal or sequel, can interfere with PGD2 signaling in the brain. The results provide also evidence that the dysfunctional DP1 network can be a target for diagnosis and intervention of NT1, a conclusion that warrants further investigations.