Figure 1.
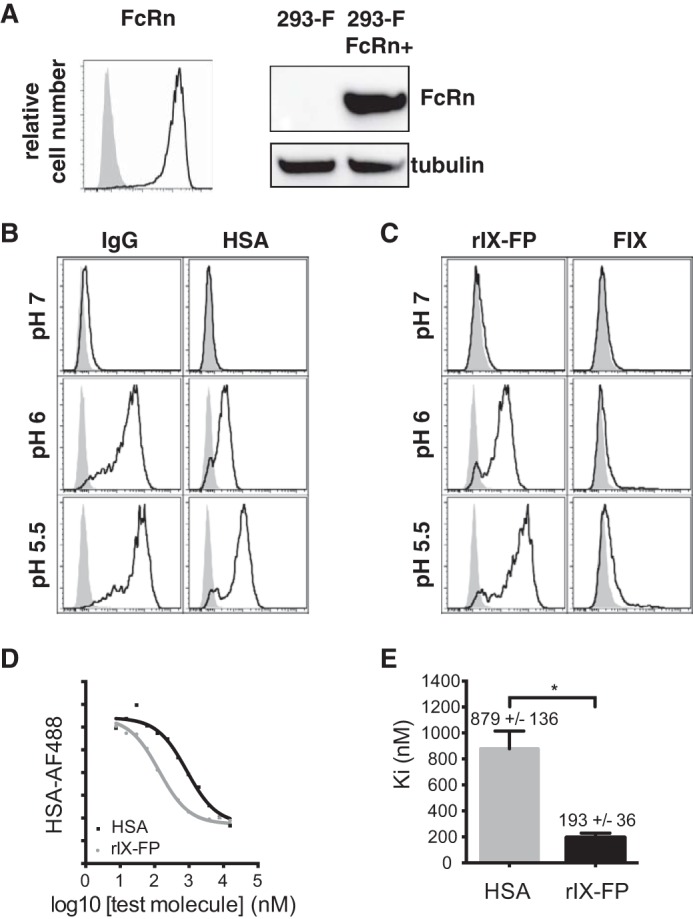
rIX-FP binds to cell-surface–expressed FcRn in a pH-dependent manner, like IgG and albumin (HSA). A, 293-F (filled gray) and 293-F FcRn+ (black outline) cells were analyzed by immunoblotting and flow cytometry analysis, using an anti-FcRn mAb. B and C, binding of cell-surface–expressed FcRn on 293-F FcRn+ cells (black outline) to fluorescently labeled IgG, albumin, rIX-FP, and rFIX was assessed at pH 5.5, 6, and 7.2. Binding to parental 293-F cells were included as negative controls (filled gray). D, relative binding of rIX-FP and albumin to cell-surface–expressed FcRn was assessed at pH 5.5 in competition-based inhibition assays, where 293-F FcRn+ cells were incubated with AF488-labeled albumin in the presence of increasing concentrations of unlabeled rHSA and rIX-FP. Data from a representative experiment are shown. E, relative binding of rIX-FP and albumin to FcRn, expressed as Ki values (nm). The data represent the means ± S.E. from four independent competition-based inhibition experiments. *, p < 0.05
