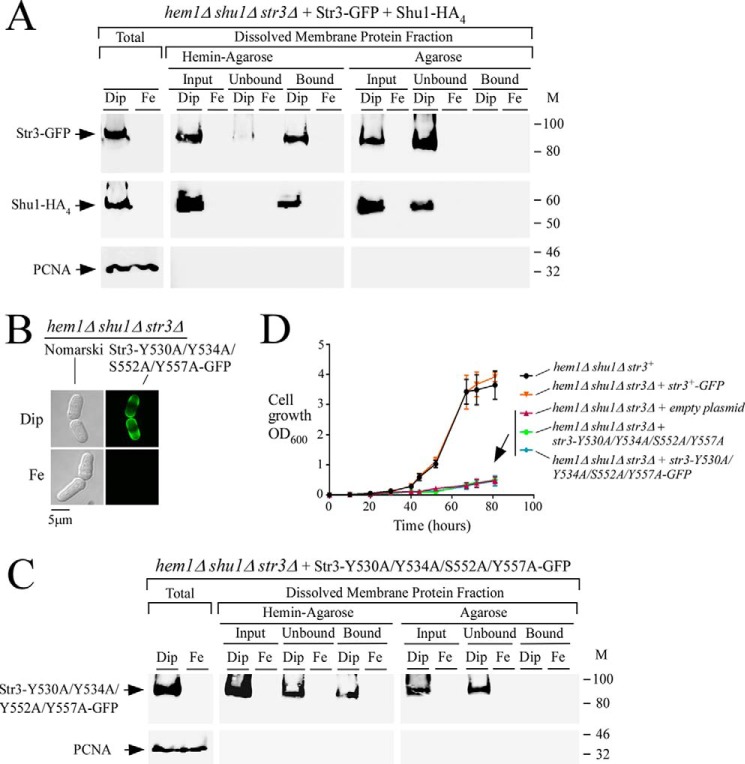
Figure 6.
Str3 binds hemin. A, hem1Δ shu1Δ str3Δ cells co-expressing GFP-tagged Str3 and HA4-tagged Shu1 were precultured in the presence of ALA (200 μm) and Dip (50 μm). Cells in the mid-exponential phase of growth were transferred to ALA-free medium and treated with Dip (250 μm) or FeCl3 (100 μm) for 3 h. Whole-cell extracts (Total) were prepared, and cell membranes were obtained by ultracentrifugation. Triton X-100–solubilized membrane proteins (input) were subjected to hemin pulldown assays using hemin-agarose or agarose alone. Unbound and bound protein fractions were analyzed by immunoblot assays using anti-GFP, anti-HA, and anti-PCNA antibodies. B, hem1Δ shu1Δ str3Δ cells expressing Str3-Y530A/Y534A/S552A/Y557A-GFP were analyzed for detection of a GFP-mediated signal by fluorescence microscopy (right). Before microscopic analysis, cells were incubated in the presence of Dip (250 μm) or FeCl3 (Fe; 100 μm) for 3 h. Nomarski optics was used to reveal cell morphology (left). C, hem1Δ shu1Δ str3Δ cells expressing GFP-tagged Str3-Y530A/Y534A/S552A/Y557A mutant protein were cultured under the same conditions as described for A. Protein fractionation, pulldown assays with hemin-agarose, and immunoblotting were carried out as indicated for A. M, positions of molecular mass of protein standards (in kDa) are indicated on the right. D, growth of the indicated strains was assessed in the presence of hemin (0.15 μm) but in the absence of ALA. Strain color codes were as follows: hem1Δ shu1Δ str3+ in black; hem1Δ shu1Δ str3Δ expressing str3+-GFP in orange; hem1Δ shu1Δ str3Δ with an empty plasmid in violet; hem1Δ shu1Δ str3Δ expressing str3-Y530A/Y534A/S552A/Y557A in green; and hem1Δ shu1Δ str3Δ expressing str3-Y530A/Y534A/S552A/Y557A-GFP in blue. Values are represented as the averages ± S.D. (error bars) of three independent experiments.