Abstract
Introduction
PCR testing at birth (‘birth-testing’) is suggested by new World Health Organization guidelines for rapid diagnosis of infants infected with HIV in utero. However there are few data on the implementation of this approach in sub-Saharan Africa and whether birth-testing affects uptake of subsequent routine early infant diagnosis (EID) testing at 6–10 weeks of age is unknown.
Methods
We reviewed 575 consecutive infants undergoing targeted high-risk birth-testing in Cape Town, South Africa, and matched those testing HIV-negative at birth (n=551) to HIV-exposed infants who did not receive birth-testing (n=551). Maternal and infant clinical and demographic data, including EID testing uptake, were abstracted from routine records.
Results
Overall 3.8% of all birth-tests conducted were positive, while later EID testing positivity rates were 0.5% for those infants testing HIV-negative at birth and 0.4% for those without birth-testing. Infants who underwent birth-testing were less likely to present for later EID compared to those without a birth-test (73% vs 85%; p<0.001). This difference persisted after adjusting for maternal and infant characteristics (adjusted odds ratio, 0.60 95% confidence interval, 0.41–0.86) and across demographic and clinical subgroups. Infants undergoing birth-testing also presented for later EID at a significantly older age (mean age 60 vs 50 days, p<0.001).
Conclusions
While the yield of targeted high-risk birth testing in this setting appears high, neonates testing HIV-negative at birth may be less likely to present for subsequent EID testing. For birth-testing implementation to contribute to overall EID programme goals, structured interventions are required to support follow-up EID services after negative birth-test results.
Keywords: HIV infection, Early infant diagnosis, Neonatal, Prevention of mother-to-child transmission, Birth testing
INTRODUCTION
Prevention of mother-to-child transmission (PMTCT) programmes have been one of the hallmarks of success in the fight against HIV/AIDS (1). In South Africa, access to antiretroviral therapy (ART) during pregnancy and infancy has steadily increased, leading to a 76% reduction in new infections among children. Currently, lifelong ART is available to all pregnant or breastfeeding women regardless of CD4 count or clinical stage of infection (2,3). Yet challenges remain in eliminating mother-to-child transmission (MTCT), with over 16,000 new paediatric infections detected in 2015 (4). It is known that those who acquire HIV in utero (IU) are at highest risk of morbidity and mortality, but strong evidence from the CHER trial indicated that prompt diagnosis and early introduction of ART can dramatically reduce mortality, disease progression and neurodevelopmental impairment in children infected with HIV (5,6,7).
The World Health Organization (WHO) recommends HIV-exposed infants be tested by six weeks of life, with those who test positive being immediately referred for initiation of ART (8,9). However, delays in result return and high levels of loss to follow up (LTFU) within the early infant diagnosis (EID) cascade may fail to link many infants to life saving ART before the peak age of mortality at 11 weeks (10,11). Although there are limited data on diagnosis and treatments of infants immediately after birth, there is consensus amongst clinicians that early initiation of ART has the potential to limit viral reservoirs and prevent disease progression early in infancy (12–14). It is also argued that as ART coverage during labour rises the proportion of vertical infections that occur IU increases relative to intra-partum (IP) infections (15). A successful birth testing regime detecting 76% of all early vertical transmissions could increase the overall proportion of infected infants linked to care, but a follow up test scheduled between 6–10 weeks of age would be required to diagnose IP and very early post-partum (PP) transmissions undetectable at birth (16,17).
South African National Guidelines recommended the use of HIV-PCR testing at birth for HIV-exposed infants at high-risk of vertical HIV transmission in 2014 (18). In mid-2015, the guidelines were further updated to include routine HIV-PCR testing at birth for all HIV-exposed infants (2).
The addition of an extra test to an already complicated cascade could result in attrition of routine EID services compared to the uptake when no birth test is available. Recent estimates indicate that only 49% of infants presented for follow-up EID testing after a negative PCR result at birth, far below the national estimates of EID coverage (19). We examined whether there is an impact from including a birth test within the EID testing protocol and looked to identify possible infant and maternal factors associated with presentation at EID testing.
METHODS
This study was a retrospective cohort study including 1,126 mother-infant dyads conducted at a single-site in the Western Cape Province, South Africa. All infants were delivered at Mowbray Maternity Hospital (MMH) between July 2013 and August 2015. MMH is a secondary-level obstetric hospital with neonatal care facilities. Of the 11,000 infants born at MMH each year, 13% are HIV-exposed and 2% are HIV-infected (14; Ref Max Kroon Personal Correspondence). Referral to a secondary care facility occurs if the pregnancy is deemed to be high-risk, due to a women’s obstetric history, general medical condition or if she presents with specific risk factors during her current pregnancy (20,21). MMH also has a primary care component allowing women with local addresses to deliver on site.
During the study period, all mothers were eligible for Option B+ (ART for all pregnant and breastfeeding women irrespective of CD4+ count). HIV-PCR testing at birth was targeted at infants identified as being at high-risk of vertical transmission as per local guidelines. High-risk status was based on maternal characteristics, primarily unsuppressed viral load, late or no initiation of ART, known non-adherence during pregnancy (clinically documented default status), or infant characteristics (symptomatic, preterm delivery or low birthweight). Birth tests occurred within 48 hours of delivery, and result return occurred prior to discharge. Provincial guidelines for the study period required all HIV-exposed infants to undergo HIV-PCR testing at routine postnatal immunization visits at 6 weeks of age. Interim HIV screening also occurred in children who presented to hospitals with opportunistic infections or symptoms related to HIV. The results of all HIV-PCR tests are hosted in the central data warehouse of the National Health Laboratory Service (NHLS), the sole provider of pathology services for the public health sector in South Africa.
All infants delivered at MMH who underwent a birth test were enrolled in the study. Infants with negative birth PCRs were matched on date of birth and mode of delivery to HIV-exposed infants who did not receive an HIV-PCR test at birth. Maternal antenatal features and obstetric characteristics of all neonates were abstracted via folder review from data collected routinely in the hospital for clinical monitoring and evaluation purposes. Folder number, name, date of birth and maternal address were used to identify the first HIV-PCR result on the NHLS system for each HIV-exposed infant before February 2016. All infants were given the complete time period of the study to return for testing.
Written informed consent was not feasible because of the retrospective nature of the research. All data was handled confidentially throughout the research process. The study, including waiver of informed consent, was approved by the Human Research Ethics Committee of University of Cape Town Faculty of Health Sciences and the Mowbray Maternity Hospital Research Committee.
Data analysis
Data were analysed using Stata Version 13.0 (Stata Corporation, College Station, Texas, USA). We described patient characteristics using summary statistics, presented using mean/median or frequencies as appropriate. Associations between categorical variables were calculated using chi-squared tests whilst the Wilcoxon rank-sum test was used for continuous variables. We defined EID presentation as mother/infant dyad accessing an HIV-PCR test after being discharged from hospital. Analyses examined the characteristics of the infants included in the study by receipt of birth testing. Logistic regression models were used to examine the independent predictors of returning for an HIV-PCR test. The results are presented as odds ratios (OR) with 95% confidence intervals (CI). Variables in the model were selected based on prior evidence and findings from descriptive statistics. We defined a secondary outcome, linkage to HIV care, as an infected infant having a specimen related to HIV care (eg. HIV-RNA, CD4, viral load, etc) sent to the NHLS for processing after the date of the positive EID assay.
RESULTS
Of 1,126 HIV-exposed infants, 575 neonates underwent birth testing, of whom 4% (22) received a positive result, 0.3% (2) returned equivocal results and 96% (551) received negative results.. The most common reason for receiving a birth test was lack of maternal ART coverage during pregnancy, associated with 50% (286) of HIV-PCR tests performed at birth (Table 1). This is followed by maternal default on treatment and high viral load which accounted for 28% (163) of infants receiving a birth test. One fifth of infants (119) were included due to low birthweight (LBW) or preterm birth. Of all infants born LBW or preterm who received a birth test, 36% (76) had evidence of inadequate maternal ART coverage (<12weeks) during pregnancy. No reason was stated for 6% (37) of infants who underwent a birth test.
Table 1. Possible reasons cited for infants receiving an HIV-PCR at birth.
Infants were given more than one possible reason for fulfilling criteria for a birth test. All reasons given for each infant were included.
| Reason For Birth Test | Frequency (N=655) | Percentage of Birth Tests (N=575) |
|---|---|---|
| No reason | 37 | 6.4% |
| ART <12 weeks | 221 | 38.4% |
| Low birthweight / preterm | 119 | 20.7% |
| High viral load | 85 | 14.8% |
| Defaulted from treatment | 78 | 13.5% |
| Unbooked | 34 | 5.9% |
| No ARVs during pregnancy | 31 | 5.4% |
| Seroconverted during pregnancy | 23 | 4.0% |
| Maternal infection | 23 | 4.0% |
| 2nd Line treatment | 20 | 3.5% |
| Needle stick injury | 3 | 0.5% |
| Substance abuse | 5 | 0.9% |
| Other | 10 | 1.7% |
Maternal infection may include: chorioamnionitis, tuberculosis, syphilis, other significant morbidity
Other may include: severe growth restriction, hepatosplenomegaly, thrombocytopaenia, congenital syphilis, cytomegalovirus or tuberculosis, respiratory distress syndrome, meconium aspiration syndrome, vocal cord paralysis, adoption, abandonment, exposure to AZT only, low maternal CD4
Infants who received a negative result at birth were matched to 551 infants who did not receive birth testing (Table 2). Of the 551 infants who did not receive a birth test, 28 were born preterm weighing >2500g, 22 weighed <2500g but were born full-term, and 22 were born preterm weighing <2500g; despite these high-risk criteria, these infants did not receive a birth test. There were no differences between the two groups with regards to infant sex, infant feeding mode or gravidity. Just over half, 50% (555) were female, and 53% (582) of all infants were delivered by normal vertex delivery. Over three quarters, 77% (843) of all mothers had previously given birth, and 82% (898) exclusively breastfed their infant. Clinical differences in the two groups could be found for maternal age, gestation and infant birthweight, which were all part of the criteria used to identify infants at high-risk of HIV transmission. Mean maternal age was 28.2 years for infants who underwent a birth test compared to 29.2 years for those infants who did not. Fewer of the 551 infants who underwent a birth test were term gestation (73% (401) vs 88% (486)) and birthweight >2500g (66% (363) vs 92% (506)) than those who did not undergo a birth test (n=551). More infants were born to mothers identifying as black African in the cohort who did not receive a birth test compared to those who did (82% (433) vs 79% (454)). Available CD4 count also varied between the two groups, but 87% (481) of CD4 data for infants who did not undergo birth testing were unavailable due to changes in hospital reporting practices.
Table 2.
Summary of population demographics by receipt of an HIV-PCR test at birth.
| Variable | Birth Test N=551 (%) |
No Birth Test N=551 (%) |
Total N=1102 (%) |
P-value |
|---|---|---|---|---|
| HIV PCR result for routine EID: | ||||
| Negative | 399 (72.4) | 468 (84.9) | 867 (78.7) | |
| Positive | 2 (0.4) | 2 (0.4) | 4 (0.4) | |
| Not tested | 150 (27.2) | 81 (14.7) | 231 (21.0) | <0.001 |
|
| ||||
| Age at routine EID testing (among infants tested) | ||||
| Mean (SD) | 59.6 (41.8) | 49.5 (22.8) | 54.1 (33.3) | <0.001 |
| 4–10 weeks | 337 (84.0) | 427 (90.9) | 764 (87.7) | |
|
| ||||
| Sex: | ||||
| Female | 279 (50.6) | 276 (50.1) | 555 (50.4) | 0.953 |
|
| ||||
| § Birthweight (g): | ||||
| Mean (SD) | 2781.4 (700.1) | 3173.5 (566.1) | 2977 (665.7) | |
| Low (<2500) | 184 (33.4) | 45 (8.2) | 229 (20.8) | <0.001 |
|
| ||||
| # Mode of Delivery | ||||
| C/S | 260 (47.2) | 260 (47.2) | 520 (47.2) | - |
|
| ||||
| § Gestation (wks) | ||||
| Preterm (<37) | 148 (26.9) | 50 (9.7) | 198 (18.0) | <0.001 |
|
| ||||
| Infant Feeding | ||||
| Breastfeeding | 438 (79.5) | 460 (83.48) | 898 (81.5) | 0.241 |
|
| ||||
| § Maternal Age (years) | ||||
| Mean (SD) | 28.2 (5.9) | 29.2 (5.3) | 28.7 (5.6) | |
| Adolescent mothers (<24) | 157 (28.5) | 102 (18.5) | 259 (23.5) | <0.001 |
|
| ||||
| Maternal population group | ||||
| Black African | 433 (78.6) | 454 (82.4) | 887 (80.5) | <0.001 |
|
| ||||
| Gravidity | ||||
| Mean (SD) | 2.5 (1.3) | 2.5 (1.2) | 2.5 (1.2) | 0.829 |
|
| ||||
| § PMTCT Coverage | ||||
| Received ART 12+weeks | v | - | 276 (50.1) | - |
| Received ART <12weeks | 218 (39.6) | - | 218 (39.6) | |
| No ART | 57 (10.3) | - | 57 (10.3) | |
|
| ||||
| § Default on Treatment | ||||
| Recorded default | 75 (13.0) | - | 75 (6.8) | - |
|
| ||||
| § Viral Load | ||||
| VL >1000 | 82 (14.2) | - | 82 (7.4) | - |
Criteria used to identify high risk infants eligible for a birth HIV PCR test.
Criteria for matching HIV-exposed infants who received a birth test to HIV-exposed infants who did not.)
Of the birth-tested infants who received a negative result at birth, 73% (401/551) presented for follow-up EID compared to 85% (470/551) of infants who did not receive an HIV-PCR test at birth and presented for routine EID, p<0.001. EID testing occurred at a significantly older age in birth-tested compared to not birth-tested infants: mean age 60 vs. 50 days, p<0.001. The proportion of positive results was lower at follow-up testing (0.5% (4/871)) than at birth (4% (21/575)). Of the 575 infants who received an HIV-PCR at birth, 21 (91%) of the 23 infants with a positive birth or routine EID test had detectable viraemia at birth signifying IU infection.
Eighty-six percent (18/21) of HIV-infected infants diagnosed at birth were linked to care. At 3 months, 17% (3/18) had an undetectable viral load and 17% (3/18) detectable but reduced viral load; 28% (5/18) of infected infants had documented viral suppression at their first virologic test at 6 months of age. 22% (4/18) of infected infants had evidence of ART initiation but no virologic data available. Routine EID testing identified four infants with HIV infection. Two of these infants had a birth test; but both lacked documented viral suppression at 12 months of age. On contrast, the two infants received only routine EID had documented viral suppression by 12 months of age (Table 3).
Table 3. HIV-PCR results for all HIV-exposed infants.
Infants deemed to be high risk of HIV-transmission underwent HIV-PCR testing at birth, NHLS database was examined to determine if a confirmatory HIV-PCR or VL was run after the initial result. Linkage to care for truly infected infants looked for a subsequent specimen sent to NHLS for processing. Routine HIV-PCR results reflect mother/infant dyad accessing an HIV DNA-PCR test after being discharged from hospital.
|
|
||||
|---|---|---|---|---|
| Routine 6 week EID | ||||
|
| ||||
| HIV-PCR result | Birth HIV-PCR (n=575) | Received birth test (n=401) | No birth test (n=470) | Total (n=871) |
| Negative result | 551 (95.7) | 399 (99.5) | 468 (99.6) | 867 (99.5) |
| Equivocal result | 2 (0.3) | - | - | - |
| Positive result | 22 (3.8) | 2 (0.5) | 2 (0.4) | 4 (0.5) |
| Possible false positive result | 1 (4.5) | - | - | - |
| Linkage to HIV care | 18 (85.7) | 2 (100) | 2 (100) | 4 (100) |
| Virally suppressed within 3 months | 3 (16.7) | - | - | - |
| Viral load <500 within 3 months | 5 (27.8) | - | - | - |
| Virally suppressed within 6 months | 5 (27.8) | - | 1 (50.0) | 1 (25.0) |
| Virally suppressed within 12 months | - | - | 1 (50.0) | 1 (25.0) |
| In care, sustained viraemia | 1 (5.6) | 1 (50.0) | - | 1 (25.0) |
| Subsequently lost from care | 4 (22.2) | 1 (50.0) | - | 1 (25.0) |
Unadjusted logistic regression analysis (Table 4) showed that only receipt of birth testing and ART coverage during pregnancy were associated with lower EID presentation. Maternal age, birthweight, gestation and population group were not associated with the outcome. From the crude analysis, it was estimated that those who received a birth test were 54% less likely to return for follow-up EID testing than those that did not receive a birth test (OR,0.46; 95%CI,0.34–0.62). The significant decrease in routine EID testing among children tested at birth persisted in multivariable analyses adjusting for maternal age, nadir CD4 cell count, ART use during pregnancy, gestational age, infant sex, maternal age, maternal population group, birthweight and infant feeding modality. Where possible, confounding factors were included in the model which reduced the association between receipt of birth testing and EID presentation: infants who received a birth test were 40% less likely to return for EID presentation than those that did not receive a birth test (AdOR,0.60; 95%CI,0.41–0.86), while those that had low ART use during pregnancy (≤12 weeks) were 31% less likely to return for EID presentation compared to those who had ≥12 weeks coverage (AdOR,0.69; 95%CI, 0.41–1.02). Women who did not receive any ARTs during pregnancy were 58% less likely to report for EID presentation compared to those that had e;12 weeks ART coverage, (AdOR,0.42; 95%CI, 0.22–0.76).
Table 4. Logistic regression analyses for predictors of EID presentation for HIV-exposed infants born at MMH in Cape Town.
Variables found to be independently associated with the outcome of EID presentation were included in the multivariate model along with variables found to be significant from previous research.
|
|
|||||
|---|---|---|---|---|---|
| Univariate Model | Multivariate Model | ||||
|
| |||||
| Risk Factors | Categories | Crude OR | 95% CI | Adjusted OR | 95% CI |
| Exposure to Birth Test | No birth test | - | - | - | |
| Received birth test | 0.46 | (0.34–0.62) | 0.60 | (0.41–0.86) | |
|
| |||||
| Sex | Male | - | - | ||
| Female | 0.92 | (0.69–1.23) | |||
|
| |||||
| Birthweight | Weight (g) | 1.00 | (0.99–1.00) | ||
| Normal birthweight | - | - | |||
| Low birthweight | 0.79 | (0.56–1.12) | |||
|
| |||||
| Maternal Age | Age (years) | 1.01 | (0.98–1.03) | ||
| Mature mother ( 24) | - | - | |||
| Adolescent mother (<24) | 0.90 | (0.62–1.29) | |||
|
| |||||
| Gestation | Gestation (weeks) | 1.00 | (0.95–1.07) | ||
| Full gestation | - | - | |||
| Preterm (<37) | 0.93 | (0.64–1.35) | |||
|
| |||||
| Prima Gravida | No | - | - | ||
| Yes | 0.88 | (0.62–1.25) | |||
|
| |||||
| Race | Black | - | - | ||
| Coloured | 0.69 | (0.43–1.09) | |||
| Foreign | 0.37 | (0.06–2.25) | |||
|
| |||||
| Infant Feeding | Breastfeeding | - | - | ||
| Formula | 1.11 | (0.75–1.66) | |||
|
| |||||
| PMTCT Coverage | Received ART 12+weeks | - | - | - | - |
| Received ART <12weeks | 0.49 | (0.35–0.69) | 0.69 | (0.41–1.02) | |
| No ART | 0.31 | (0.18–0.54) | 0.42 | (0.22–0.76) | |
|
| |||||
| Default on Treatment | No default recorded | - | - | ||
| Recorded default | 0.94 | (0.52–1.69) | |||
|
| |||||
| Viral Load | VL <1000 | - | - | ||
| VL >1000 | 0.70 | (0.42–1.17) | |||
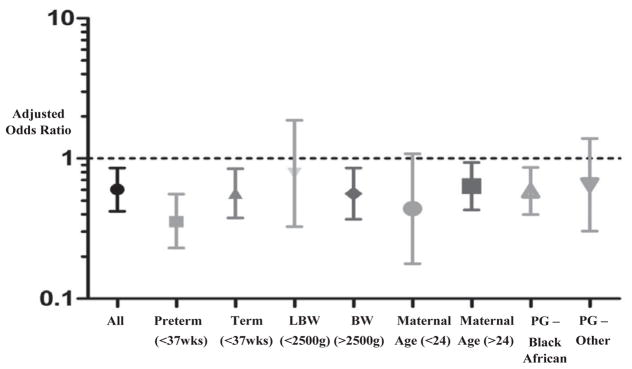
We also assessed the effect size in different subgroup populations (Figure 1). The trend of reduced EID presentation after receipt of a negative test result at birth persisted across subgroup populations. Those born with LBW (n=229), preterm (n=198) or born to mothers with younger maternal age (n=259) or who did not identify as black African (n=124) had the greatest influence on the association between receipt of a birth test and EID presentation; however, all influential subgroups had small populations (n<260) with wide confidence intervals often spanning 1. In order to further assess these effects, we conducted a sensitivity analyses on 826 mother-infant dyads, excluding preterm and LBW infants, who have the highest risk of morbidity and mortality, but were also more likely to undergo birth testing. As these infants may have died before routine EID testing could take place, they were excluded from both groups for this subgroup analysis. The negative association between birth testing and EID presentation increased (AdOR, 0.54 95% CI 0.34–0.86).
Figure 1.
The adjusted odds ratio for EID presentation having received an HIV-PCR test at birth is shown in multiple subgroup populations. In each subgroup analysis the population was restricted to include infants only with the desired population. Regression analysis was then performed to estimate the association within each subgroup controlling for confounding variables.
(Abbreviations: LBW: low birthweight, BW: birthweight, Maternal Age: Years, PG: Population Group)
DISCUSSION
Two key findings emerged from this novel estimation of routine EID test coverage of HIV-exposed infants who underwent birth testing in sub-Saharan Africa. First, targeted birth testing successfully identified mothers at high-risk for transmission to their infants, with diagnosis of HIV within 2 days of life possible for 92% of all infected infants who returned for complete EID testing. Second, neonates undergoing HIV testing at birth appeared less likely to receive subsequent EID testing compared to infants who did not receive a birth test.
We found routine EID coverage to be higher than national estimates of 73%, with 85% of HIV-exposed infants who did not receive a birth test (estimated to be 75% of all deliveries), and 73% of those who received a birth test, returning for routine EID testing (25). For HIV-infected infants, levels of linkage to care were significantly lower for infants diagnosed at birth (100% vs. 86%). However, infants diagnosed at birth were more likely to achieve viral suppression at 6 months than infants diagnosed from routine EID testing. The lower levels in overall linkage to care could be explained by survival bias; infants infected in utero are at higher risk of rapid disease progression, and infants born preterm or with a LBW were more likely to receive an HIV-PCR test at birth but have 6 fold increased risk of neonatal mortality (26,27). Infected infants may therefore have died before possible linkage to care could occur.
Although the attendance at routine EID testing was higher than national estimates, the negative association between birth testing and EID presentation persisted through all sensitivity analysis. Unlike other studies looking at retention within the EID cascade, only receipt of an HIV-PCR test at birth and low ART coverage during pregnancy were associated with EID presentation. Predictors such as maternal age, WHO clinical stage, parity and preterm delivery were not associated (24,28–30). During the study period, HIV-PCR testing at birth was targeted at infants thought to be at high-risk of HIV transmission. Half of birth tests were attributed to limited ART coverage during pregnancy, which was predictive of lower EID presentation. While the reasons behind this finding require further investigation, it is possible that low ART coverage could be a proxy for reduced antenatal visits and suboptimal patient care-seeking behaviour. It may suggest that risk factors associated with being at high-risk of perinatal HIV transmission require special consideration around retention within the PMTCT/EID cascades.
Our data have demonstrated that birth testing could lead to a higher yield of positive infants being initiated on treatment earlier if implemented correctly, but including a birth test in the EID algorithm adds additional steps into an already complicated cascade. Research has shown each step in the cascade to be an opportunity for LTFU to occur, and it is known that despite continuing transmission risk from breastfeeding in the postpartum period (late postpartum infections can account for 8–25% of HIV-infections in infants) there is extensive loss to follow up after an initial HIV test, with very few infants presenting for testing after the cessation of breastfeeding (31,32). Receiving a negative result immediately after delivery may cause confusion around the need for further testing at 6 weeks, especially considering the likelihood that caregivers who receive a negative HIV result are significantly less likely to receive follow up counseling compared to those with a positive result (34).
PMTCT services are designed to offer support to women living with HIV and provide them with information for both antenatal and postnatal care; however, a report by the WHO details how counseling is often provided by busy, overworked staff members, who focus only on the initial HIV test result and ART treatment (2,35,36). Evidence has suggested that women living with HIV in South Africa have inadequate knowledge of HIV transmission during the post-partum period and are unaware of EID testing schedules (37). There is a clear need for further emphasis on early infant follow-up, along with ensuring accurate health records and strong data management to enable timely identification of infants who remain HIV-exposed and require EID testing. Where appropriate testing schedules, counseling curriculum and PMTCT services may need to be revised in order to integrate birth testing into the EID cascade.
While the negative association between birth testing and EID presentation is of clear concern given recent policy changes within South Africa, the results have several limitations. First, generalizations regarding other settings and facility levels should be made with caution. The results reflect a single urban setting within South Africa. All participants were enrolled in a referral obstetric unit within a maternity service in Cape Town and required post discharge referral back to the referring unit with consequent opportunity for communication failure and LTFU. Second, due to the retrospective nature of the research, we were unable to collect social and demographic variables such as HIV knowledge, employment and education of mothers that could have provided interesting insight into further predictors of EID presentation. We were unable to ascertain the proportion of LTFU caused by infant mortality, and why mother/infant dyads would otherwise become disengaged from care. Qualitative studies focused on finding mothers lost from care and exploring why they did not report for testing would provide greater understanding to the issues surrounding LTFU within the EID cascade. We were also unable access the process of result return to caregivers, meaning that levels of LTFU could be underestimated. Finally, during the study period HIV-PCR testing at birth was targeted at infants thought to be at high-risk of perinatal transmission. It would be important to repeat the study in the light of the introduction of universal birth testing to examine further predictors of LTFU after birth testing and reflect on the impact of targeted birth testing in this setting.
In conclusion, these results show that targeted birth testing in South Africa is extremely effective at identifying infected infants. Birth testing has the potential to improve infant survival by increasing earlier access to ART for infants infected IU who are most at risk of early death. However, there is a danger of reducing population coverage of routine EID services at later ages for infants testing negative at birth. Consequently, HIV birth testing programmes should be implemented with recognition of the potential advantages and challenges involved, and further research should consider the optimal implementation of such programmes while reflecting on the programmatic impact of changing current testing algorithms.
Acknowledgments
This research was supported in part by the Elizabeth Glaser Pediatric AIDS Foundation.
Footnotes
Conflicts of Interest: None declared
References
- 1.World Health Organization. Geneva, Switzerland: 2015. Progress report on the global plan towards the elimination of new HIV infections among children and keeping their mothers alive. Available at: http://www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf. [Google Scholar]
- 2.Republic of South Africa: Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents, and adults. Pretoria, South Africa: 2014. Available at: http://www.sahivsoc.org/upload/documents/HIV%20guidelines%20_Jan%202015.pdf. [Google Scholar]
- 3.United Nations Joint Programme on AIDS. The Gap Report. Geneva, Switzerland: 2014. p. 422. Available from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf. [Google Scholar]
- 4.Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, et al. GBD 2015 HIV Collaborators. Lancet HIV. 2016. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015; pp. 1005–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayaux MJ, Burgard M, Teglas JP, Cottalorda J, Krivine A, Simon F, et al. Neonatal characteristics in rapidly progressive perinatally acquired HIV-1 disease. The French Pediatric HIV Infection Study Group. JAMA. 1996 Feb 28;275(8):606–10. [PubMed] [Google Scholar]
- 6.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008 Nov 20;359(21):2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012;26:1685–1690. doi: 10.1097/QAD.0b013e328355d0ce. http://dx.doi.org/10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barron P, Pillay Y, Doherty T, Sherman G, Jackson D, Bhardwaj S, et al. Eliminating mother-to-child HIV transmission in South Africa. Cómo Elimin la Transm del VIH la madre al niño en Sudáfrica [Internet] 2013;91(1):70–4. doi: 10.2471/BLT.12.106807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Consolidated guidelines on the use of antiretrovirals for the treatment and prevention of HIV infection: Recommendations for a public health approach. 2. Geneva: 2015. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html. [Google Scholar]
- 10.Bourne DE, Thompson M, Brody LL, Cotton M, Draper B, Laubscher R, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. Aids. 2009;23(1):101–6. doi: 10.1097/qad.0b013e32831c54bd. [DOI] [PubMed] [Google Scholar]
- 11.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: A pooled analysis. Lancet. 2004;364(9441):1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 12.Ananworanich J, Robb ML. The transient HIV remission in the Mississippi baby: Why is this good news? J Int AIDS Soc. 2014;17:1–2. doi: 10.7448/IAS.17.1.19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman G. HIV testing during the neonatal period. S Afr J HIV Med. 2015;16(1):2–4. doi: 10.4102/sajhivmed.v16i1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies M-A, May M, Bolton-Moore C, Chimbetete C, Eley B, Garone D, et al. Prognosis of Children with HIV-1 Infection Starting Antiretroviral Therapy in Southern Africa: A Collaborative Analysis of Treatment Programs. Pediatr Infect Dis J. 2015;33(6):608–16. doi: 10.1097/INF.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn L, Kroon M. Breastfeeding and the 2015 South African guidelines for prevention of mother-to-child transmission of HIV. S Afr J HIV Med. 2015;16(1):5. doi: 10.4102/sajhivmed.v16i1.377. Art. #377. http://dx.doi.org/10.4102/sajhivmed.v16i1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilian RR, Kalk E, Bhowan K, Berrie L, Carmona S, Technau K, et al. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6 weeks of age. J Clin Microbiol. 2012 Jul;50(7):2373–7. doi: 10.1128/JCM.00431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lilian RR, Kalk E, Technau K-G, Sherman GG. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis Journalnfectious Dis J [Internet] 2013;32(10):1080–5. doi: 10.1097/INF.0b013e318290622e. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23574775. [DOI] [PubMed] [Google Scholar]
- 18.Republic of South Africa: Department of Health. Pretoria, South Africa: 2014. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents, and adults. Available at: http://www.sahivsoc.org/upload/documents/HIV%20guidelines%20_Jan%202015.pdf. [Google Scholar]
- 19.Maritz J, Hsiao N, Presier W, Myer L. Low Uptake of Routine Infant Diagnostic Testing Following HIV PCR Testing at Birth. Conference of Retroviruses and Opportunistic Infections; 2016; Poster Presentation. [Google Scholar]
- 20.Department of Health SA. Guidelines for Maternity Care in South Africa 2Department of Health, S. A. Guidelines for Maternity Care in South Africa 2007: A manual for clinics, community health centres and district hospitals, 1–173.007: A manual for clinics, community heal. 2007;2015:1–173. [Google Scholar]
- 21.Kroon M. Personal Communication. University of Cape Town; 2016. [Google Scholar]
- 22.Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: New global challenges in the era of paediatric HIV elimination. Lancet Infect Dis. 2016;16(6):e92–107. doi: 10.1016/S1473-3099(16)00055-4. [DOI] [PubMed] [Google Scholar]
- 23.Leshabari SC, Blystad A, de Paoli M, Moland KM. HIV and infant feeding counselling: challenges faced by nurse-counsellors in northern Tanzania. Hum Resour Health. 2007;5:18. doi: 10.1186/1478-4491-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarus R, Struthers H, Violari a. Hopes, fears, knowledge and misunderstandings: responses of HIV-positive mothers to early knowledge of the status of their baby. AIDS Care. 2009;21(3):329–34. doi: 10.1080/09540120802183503. [DOI] [PubMed] [Google Scholar]
- 25.Woldesenbet S, Goga A, Jackson D. The South African Programme To Prevent Mother-To-Child Transmission Of Hiv (Pmtct) Evaluation Ofthe Early Infant Diagnosis Service In Primary Health Care Facilities In South Africa: Report On Results Of A Situational Assessment. 2012 Available from: http://www.nhls.ac.za/assets/files/Situationalassessmentreportfinal_10thOct12.pdf.
- 26.Slyker JA, Patterson J, Ambler G, Richardson BA, Maleche-Obimbo E, Bosire R, et al. Correlates and outcomes of preterm birth, low birth weight, and small for gestational age in HIV-exposed uninfected infants. BMC Pregnancy Childbirth. 2014;14:7. doi: 10.1186/1471-2393-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro RL, Lockman S. Mortality among HIV-Exposed Infants: The First and Final Frontier. Clin Infect Dis. 2010;50(3):445–7. doi: 10.1086/649887. [DOI] [PubMed] [Google Scholar]
- 28.Hassan AS, Sakwa EM, Nabwera HM, Taegtmeyer MM, Kimutai RM, Sanders EJ, et al. Dynamics and constraints of early infant diagnosis of HIV infection in rural Kenya. AIDS Behav. 2012;16(1):5–12. doi: 10.1007/s10461-010-9877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woldesenbet SA, Jackson D, Goga AE, Crowley S, Bs MB, Doherty T, et al. Missed Opportunities for Early Infant HIV Diagnosis: Results of A National Study in South Africa. J Acquir Immune Defic Syndr. 2015;68(3):26–32. doi: 10.1097/QAI.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mnyani CN, Simango A, Murphy J, Chersich M, McIntyre JA. Patient factors to target for elimination of mother-to-child transmission of HIV. Global Health. 2014;10(1):36. doi: 10.1186/1744-8603-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciaranello AL, Park J-E, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med [Internet] 2011;9(1):59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sibanda EL, Weller IVD, Hakim JG, Cowan FM. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013;27(17):2787–97. doi: 10.1097/QAD.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mor Z, Chemtob D, Pessach N, Nitzan-Kaluski D. Human immunodeficiency virus in new born of infected mothers: Pregnancy, breastfeeding and prevention. Harefua. 2006;145:670. [PubMed] [Google Scholar]
- 34.Sawe HR, Mfinanga JA, Ringo FH, Mwafongo V, Reynolds TA, Runyon MS. HIV counselling and testing practices for children seen in an urban emergency department of a tertiary referral hospital in Dar es Salaam, Tanzania: a retrospective cross-sectional study. BMJ Open. 2016;6(2):e010298. doi: 10.1136/bmjopen-2015-010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A, Singh B, Kusuma YS. Counselling services in prevention of mother-to-child transmission (PMTCT) in Delhi, India: An assessment through a modified version of UNICEF-PPTCT tool. J Epidemiol Glob Health. 2015;5(1):3–13. doi: 10.1016/j.jegh.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rujumba J, Neema S, Tumwine JK, Tylleskar T, Heggenhougen HK. Pregnant women’s experiences of routine counselling and testing for HIV in Eastern Uganda: a qualitative study. BMC Health Serv Res. 2013;13:189. doi: 10.1186/1472-6963-13-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woldesenbet SA, Jackson D, Goga AE, Crowley S, Doherty T, Mogashoa MM, et al. Missed opportunities for early infant HIV diagnosis: results of a national study in South Africa. J Acquir Immune Defic Syndr. 2015;68(3):e26–32. doi: 10.1097/QAI.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]