Figure 1.
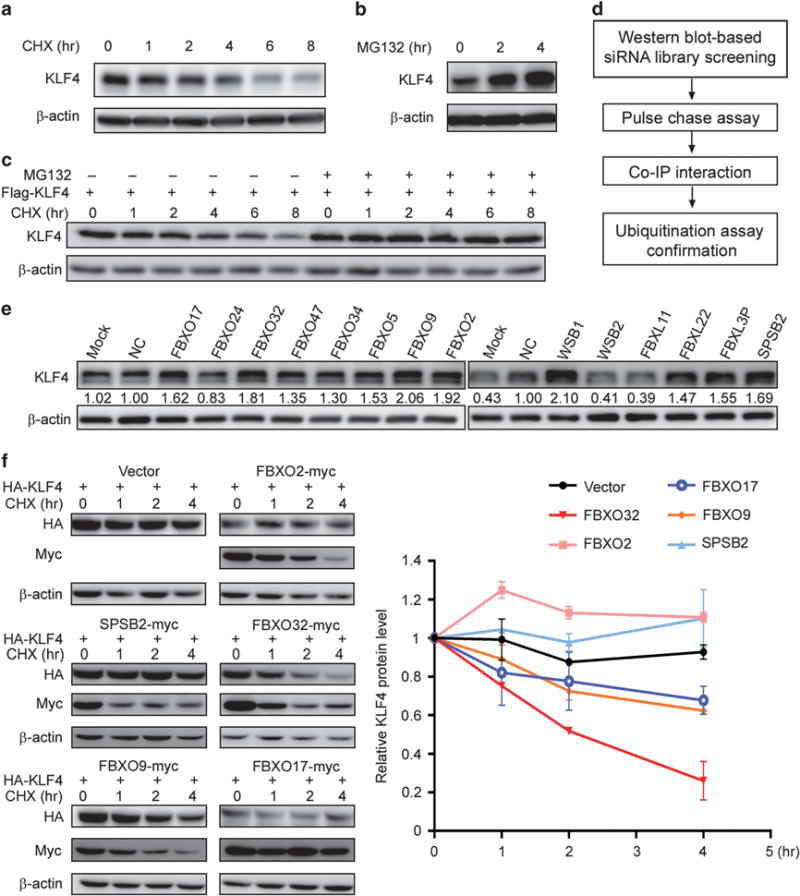
A genome-wide functional screen for E3 ubiquitin ligase(s) targeting KLF4 protein for degradation. (a, b) KLF4 protein has a short half-life and is degraded via ubiquitin–proteasome pathway. MCF7 cells were plated in six-well plates. Twenty-four hours later, the cells were treated with 50 μM CHX (a) or 20 μM MG132 (b) for the indicated time. KLF4 level was analyzed by western blot. (c) Exogenously expressed KLF4 is degraded through proteasomal pathway. HEK293T cells were transfected with Flag-tagged KLF4, treated with 50 μM CHX for indicated time with or without 20 μM MG132. The exogenous KLF4 was probed by immunoblot with Flag antibody. (d) Experimental procedure flow chart for identification of the E3 ubiquitin ligase(s) targeting KLF4 protein. (e) A second round of siRNA screening was carried out to confirm the candidates. KLF4 level was probed by immunoblot after siRNA knockdown of 14 candidates. Quantification was made by Image J (NIH, Bethesda, MD, USA). (f) FBXO32 facilitates KLF4 degradation. HA-tagged KLF4 is co-transfected with five E3 ligase candidates individually. Twenty-four hours after transfection, the cells were treated with CHX for indicated time. KLF4 and E3 ligase candidates were analyzed by western blot with antibodies against HA and myc. Quantification was made by Image J (NIH) and the statistics were done with GraphPad Prism.
