Figure 2.
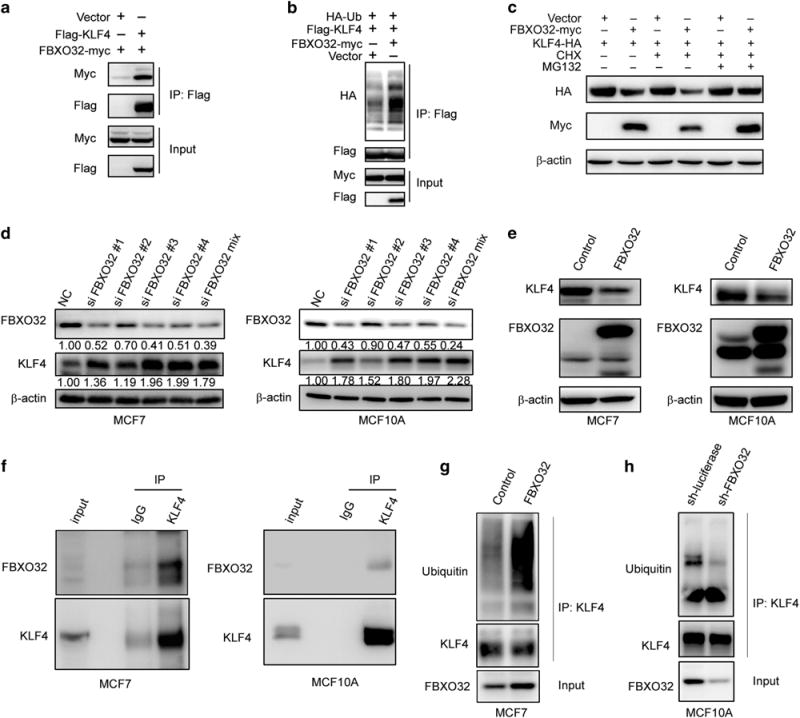
FBXO32 interacts with KLF4 and mediates KLF4 ubiquitination and degradation. (a) Exogenous FBXO32 interacts with KLF4. HEK293T cells were transfected with Flag-tagged KLF4 and either empty vector or the Myc-tagged FBXO32. Twenty-four hours after transfection, the cells were treated with 20 μM MG132 for 4 h. The co-IP experiment was performed using anti-Flag M2 affinity gel. The immunoprecipitates were analyzed by western blot with anti-Myc antibody. (b) FBXO32 increases exogenous KLF4 ubiquitination. Indicated plasmids were transfected into HEK293T cells. Twenty-four hours after transfection, the cells were treated with 20 μM MG132 for 6 h to accumulate the ubiquitinated KLF4. Anti-Flag M2 affinity gel was used to immunoprecipitate tagged KLF4. The ubiquitin was probed by HA-specific antibody to reflect the ubiquitination of KLF4. (c) MG132 restores FBXO32-mediated KLF4 decreasing. HA-tagged KLF4 is transfected with either the empty vector or the Myc-tagged FBXO32 in HEK293T cells. Twenty-four hours after transfection, CHX and MG132 were added as indicated for 4 h. The cell lysates were subjected to western blot to evaluate the protein level of KLF4. (d) FBXO32 knockdown leads to KLF4 protein accumulation. siRNAs specifically targeting FBXO32 are transfected into MCF7 and MCF10A cells, the expression of KLF4 and FBXO32 was tested by western blot. (e) FBXO32 overexpression decreases the protein level of KLF4. MCF7 and MCF10A cells stably expressed FBXO32 were established. The cell lysates with equal amount of total protein were used to test the protein levels of FBXO32 and KLF4 via immunoblotting. (f) Endogenous FBXO32 interacts with KLF4. MCF7 or MCF10A cells cultured in 10 cm dish were treated with MG132 for 4 h and harvested in 1 ml of cell lysis buffer. IP was performed with anti-KLF4 primary antibody. The immunoprecipitates were analyzed by western blot and detected with anti-FBXO32 antibody. (g, h) Effect of FBXO32 on the ubiquitination of endogenous KLF4. Control and FBXO32 overexpressed MCF7 cells (g) or control and FBXO32 knockdown MCF10A cells (h) were treated with MG132 for 6 h and subjected to ubiquitination assay. Immunoprecipitated KLF4 proteins were analyzed with western blot and detected with ubiquitin antibody.
