Figure 3.
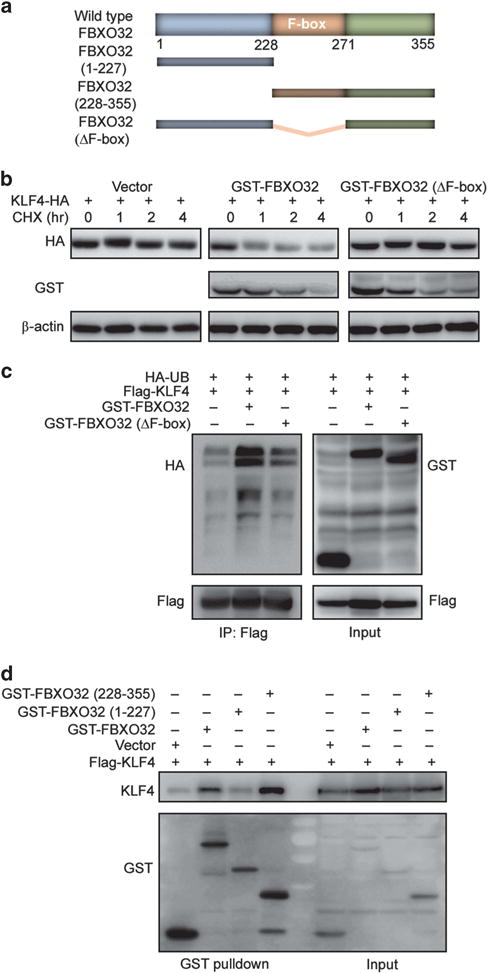
The C-terminus of FBXO32 is responsible for recognizing and interacting with KLF4 and the F-box domain is critical for FBXO32 E3 ligase function. (a) Schematic of human FBXO32 with previously identified domains as well as deletion mutants generated in the present work. (b) The F-box domain is critical for the E3 ligase activity of FBXO32. HA-tagged KLF4 and GST-tagged FBXO32 were transfected into HEK293T cells. Twenty-four hours after transfection, the cells were treated with CHX for indicated time. The cell lysates were analyzed by western blot for KLF4 protein level. (c) Deletion of F-box abrogated FBXO32-mediated KLF4 ubiquitination. Flag-tagged KLF4 and GST-tagged wild-type or F-box deletion mutant FBXO32 were co-transfected into HEK293T cells. The cells were treated with MG132 and subjected to ubiquitination assay as described. (d) FBXO32 interacts with KLF4 via its C-terminus. Indicated plasmids were transfected into HEK293T cells. Twenty-four hours later, the cells were treated with MG132 and subjected to GST pulldown. The precipitates were analyzed by western blot.
