Figure 1.
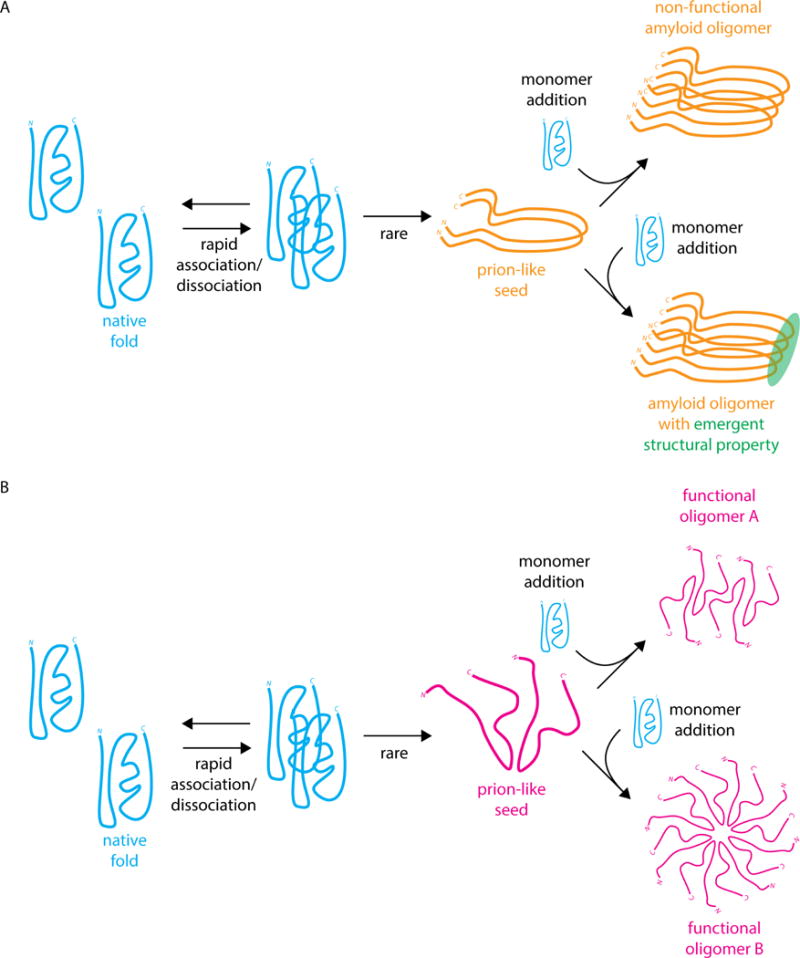
(A) Classical amyloid prion formation by nucleation of a prion-like seed followed by recruitment of monomers to the prion-like conformation to generate a cross-beta sheet amyloid oligomer [12]. The amyloid oligomer could be non-functional (top), as in the [PSI+] prion, or gain an emergent structural property (bottom), as in the HET-s prion [13] (B) Hypothetical mechanism of prion-like oligomerization leading to two distinct prion-like oligomers that maintain their molecular activity and are not amyloid in nature.
