Abstract
Background
Neurotoxoplasmosis is a common opportunistic infection in HIV/AIDS patients. Imaging identification of neurotoxoplasmosis assists in timely treatment.
Purpose
To delineate the frequency of imaging abnormalities in patients with neurotoxoplasmosis on different MR sequences with a particular focus on SWI, and NCCT.
Material and methods
The PACS database was retroactively searched over a 5-year period for patients with neurotoxoplasmosis who underwent MRI with SWI. Included patients had imaging features of neurotoxoplasmosis based on consensus review by two neuroradiologists, a clinical diagnosis of neurotoxoplasmosis at the time of MRI, and diagnostic confirmation based on positive serum or CSF serology or histopathology; 15 patients were included. The number of abnormal foci with restricted diffusion, increased FLAIR signal, intrinsic T1 hyperintensity, abnormal enhancement (CE-T1WI), and intrinsic hyperdensity on CT were recorded.
Results
Intralesional susceptibility signal (ISS) foci on SWI were observed in 93.3% of patients with neurotoxoplasmosis (mean size 5.2 ± 3.8 mm). The average number of ISS foci was 3.9 per patient; 3/15 (20.0%) had a single ISS. Amongst other MR sequences, hyperintense FLAIR foci were the most common abnormalities observed (12.4 lesions/patient), followed by enhancing foci (8.2 lesions/patient), foci of restricted diffusion (7.1 lesions/patient), and intrinsic T1 hyperintense foci (3.4 lesions/patient). Abnormalities were least frequently observed on NCCT: abnormalities were identified in 5/15 (33.3%) patients, at a rate of 0.4 lesions/patient.
Conclusion
ISS foci are present in the vast majority of neurotoxoplasmosis patients, likely representing hemorrhage. The incidence and frequency of other abnormal foci are highest on FLAIR, and lowest on NCCT.
Keywords: Toxoplasmosis, HIV, AIDS
1. Introduction
Neurotoxoplasmosis has become a leading cerebral opportunistic infection in patients with HIV [1]. It is estimated that nearly 40% of the HIV-infected population will develop neurological manifestations during their lifetime, while over 75% are affected on autopsy [[2], [3], [4], [5]]. In addition, one-third of patients with advanced immunosuppression may develop cerebral toxoplasmosis in a 12-month period [6].
The initial diagnosis of neurotoxoplasmosis is usually made empirically if multiple ring-enhancing lesions are present on brain MRI, serology is positive for Toxoplasma gondii in the CSF or blood, and subsequent clinico-radiological improvement after anti-toxoplasma therapy is observed. However, a shorter time to diagnosis is preferable in order to avoid the side effects of anti-toxoplasma medications, as well as to avoid treatment delays if the alternative scenario of tumor is present. Additionally, empiric diagnosis can be problematic because elevated serum IgG anti-toxoplasma titers are common in the general population in the absence of active disease [[6], [7], [8], [9]]. While brain biopsy is the gold-standard for diagnosis, it is typically reserved for patients who are seronegative and have shown a lack of improvement after a two-week trial of empiric therapy [10]. Meanwhile, the use of steroids in tandem with anti-toxoplasma medications can falsely improve the neurological symptoms in patients thought to have cerebral toxoplasmosis, but who actually have PCNSL [11].
Already, a number of imaging features of neurotoxoplasmosis have been described. The process often appears as multifocal ring-enhancing lesions in the cortex and periventricular white matter; when present, an “eccentric target sign” consistenting of an eccentric nodule along the rim of an enhancing lesion is considered pathognomonic [12]. However, to date only a few case reports have recently described abnormal signal within neurotoxoplasmosis lesions on CT or GRE T2*WI MRI [13,14]. SWI utilizes blood oxygenation level-dependent principles to detect susceptibility effects via the paramagnetic properties of hemorrhage, venous blood, or physiologic iron, as well as to identify calcium [[15], [16], [17], [18], [19]]. SWI has already proven to be much more sensitive than CT or conventional GRE T2*WI in the detection of cerebral hemorrhage [15,20]. Hence, the aim of this study was to identify the frequency of abnormal SWI foci in patients with neurotoxoplasmosis, as well as to delineate the frequency of abnormalities on other MR imaging sequences and non-contrast CT (NCCT).
2. Materials and methods
2.1. Patient selection and diagnosis
The institutional review board approved this retrospective study. During a 5-year period from 6/2010 to 6/2015, the electronic health records and PACS database were searched for patients with neurotoxoplasmosis who underwent SWI MRI at presentation. The inclusion criteria for neuroimaging review were: 1) a recent clinical diagnosis of neurotoxoplasmosis with a brain MRI with intravenous gadolinium contrast agent that utilized SWI, and 2) confirmation via either clinical improvement after therapy, positive serology, or histopathological diagnosis. Patients were excluded due to either: 1) lacking an MRI prior to therapy, or 2) suboptimal and/or non-diagnostic MRI secondary to motion or artifacts.
2.2. Diagnostic criteria
The etiologic workup of those patients ultimately determined to have neurotoxoplasmosis included detection of serum or CSF IgG+/− IgM antibodies against T. gondii. Despite T. gondii serology testing, a CSF analysis was also obtained at admission for exclusion of other less common CNS opportunistic organisms, including, but not limited to: JC virus, herpes simplex virus type 1 and 2, varicella zoster virus, Cryptococcus neoformans, and Mycobacterium tuberculosis.
Brain MRI were interpreted via consensus of a neuroradiology fellow (GMC) and a staff neuroradiologist with >10 years’ experience (AMM). To assess the frequency of brain lesions in patients with neurotoxoplasmosis, the readers documented the frequency of neuroimaging findings by manually counting the number of lesions on the following sequences at initial presentation: 1) SWI, 2) FLAIR, 3) DWI, 4) noncontrast T1WI, 5) CE-T1WI, and 6) NCCT. The maximum number of lesions was recorded as 20, even if greater. Each sequence was reviewed in conjunction with FLAIR, which was considered the standard for the presence or absence of a lesion. Regarding SWI, both the axial 2mm-thick and reformatted minimum intensity projections (8–10 mm thickness) were reviewed in conjunction with filtered-phase maps in order to distinguish paramagnetic (e.g. ferritin, hemosiderin) from diamagnetic (e.g. calcification) substances. Intralesional susceptibility signal (ISS) foci were defined as blooming signal on susceptibility-weighted images corresponding with abnormal foci on other sequences. ISS were excluded if the pattern and distribution of the hemorrhagic foci had imaging characteristics of vasculopathy-related microhemorrhages as multiple scattered punctate foci or confluent patchy subcortical and periventricular signal changes on FLAIR images, rather than ill-defined toxoplasmosis lesions surrounded by perilesional edema. In addition, the abnormalities within FLAIR-positive lesions on the following imaging sequences were noted: 1) the number of enhancing lesions on CE-T1WI, 2) the number of foci of reduced diffusion on DWI and ADC images, 3) the number of hyperintense foci on noncontrast T1WI, and 4) the number of hyperdense foci on NCCT. Size of ISS was recorded in mm; size and characteristics of abnormal foci on other sequences was not collected.
MR examinations were acquired on both 3T ((Tim Trio; Siemens Healthcare, Erlangen, Germany) with the following sequence parameters: TR 27 ms, TE 20 ms, flip angle 15°, bandwidth 250 kHz, matrix size 256 × 134, thickness 2 mm, parallel factor = 2, and acquisition time of about 2.5 min) and 1.5T ((Avanto; Siemens Healthcare, Erlangen, Germany) with the following sequence parameters: TR 49 ms, TE 40 ms, flip angle 15°, bandwidth 80 kHz, matrix size 256 × 157, slice thickness 2.5 mm, parallel factor = 2, and an acquisition time of about 3 min) scanners, being obtained on four different scanner models over the study period. CT imaging was completed on either a 64 multidetector scanner (Sensation, Siemens Healthcare, Erlangen, Germany), or a dual-source 256 slice scanner (Definition Flash, Siemens Healthcare, Erlangen, Germany). Regarding postcontrast CE-T1WI MRI, the intravenous gadolinium-based contrast utilized was gadobutrol, with a weight-based bolus of 0.1 mL/kg (0.1 mmol/kg, maximum of 10 mL total), and a 5-min delay prior to T1WI acquisition. Each sequence was obtained in multiple planes at 5 mm axial thickness, with the exclusion of SWI, which was reconstructed at 2 mm. The DWI gradient strength was b = 1000 s/mm2.
3. Results
3.1. Patient population
The electronic health records and PACS database searches yielded 32 patients with toxoplasmosis who underwent contrast enhanced MRI with SWI; 17 were excluded from imaging review due to SWI not being performed (n = 9), a lack of clinical or MRI followup (n = 7), or motion degradation during the MRI that precluded interpretation (n = 1). Ultimately, 15 patients (13 males) were included for imaging review (mean age 44 years, range 34–51). All 17 patients were immunocompromised with HIV/AIDS. Every patient had CD-4 T-cell count levels <50 cells/uL (mean: 7.2 cells/uL; range 1.3–26.0). Average time to followup was 15 months for MRI, and 20 months for clinical followup.
3.2. Clinical followup
Three patients underwent biopsy; two of these patients had improvement on an MRI within 2 weeks of initiating anti-toxoplasma therapy, while the third had cerebellar enhancing lesions that did not improve until nearly 2 months after starting anti-toxoplasma therapy (Table 1). None of the patients expired prior to the resolution of their neurological manifestations. Two patients expired from non-related processes during the 5-year study period: one from aspergillus pneumonia, and the other from an unknown cause.
Table 1.
MR, CT, and clinical data on 15 patients with neurotoxoplasmosis. HIV+ indicates infected patients wit low CD4 counts (below 50 uL). MR indictes the magnet strength used at the time of the study acquisition.
| No./Sex/Age(y) | FLAIR | CE-T1WI | DWI | SWI | T1WI | CT | HIV+ | Biopsy | MR |
|---|---|---|---|---|---|---|---|---|---|
| 01/M/34 | 20 | 20 | 30 | 3 | 0 | 1 | Y | Y | 3.0T |
| 02/M/46 | 20 | 20 | 20 | 5 | 0 | 1 | Y | N | 3.0T |
| 03/M/35 | 20 | 20 | 12 | 4 | 16 | 0 | Y | N | 3.0T |
| 04/M/44 | 20 | 20 | 7 | 20 | 0 | 0 | Y | N | 3.0T |
| 05/M/37 | 20 | 20 | 5 | 1 | 9 | 1 | Y | N | 1.5T |
| 06/F/40 | 20 | 5 | 20 | 2 | 1 | 0 | Y | N | 3.0T |
| 07/M/40 | 20 | 4 | 3 | 3 | 11 | 0 | Y | N | 1.5T |
| 08/M/42 | 20 | 0 | 11 | 10 | 9 | 0 | Y | Y | 3.0T |
| 09/F/50 | 10 | 1 | 0 | 0 | 1 | 1 | Y | N | 1.5T |
| 10/M/48 | 5 | 5 | 1 | 4 | 2 | 0 | Y | N | 3.0T |
| 11/M/50 | 5 | 2 | 2 | 1 | 0 | 2 | Y | N | 3.0T |
| 12/M/50 | 2 | 2 | 2 | 2 | 1 | 0 | Y | N | 3.0T |
| 13/M/39 | 2 | 2 | 0 | 2 | 0 | 0 | Y | N | 3.0T |
| 14/M/51 | 1 | 1 | 1 | 1 | 1 | 0 | Y | N | 1.5T |
| 15/M/43 | 1 | 1 | 1 | 1 | 0 | 0 | Y | N | 1.5T |
3.3. Imaging results
ISS on SWI were identified in 14/15 (93.3%) of the neurotoxoplasmosis patients (mean size 5.2 ± 3.8 mm) (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5). For all patients, the average number of ISS was 3.9 lesions/patient; 4/15 (26.7%) patients had a solitary ISS. Other MR and CT characteristics of the toxoplasmosis lesions are provided in Table 1. The average number of abnormal foci identified on the various imaging sequences were as follows: SWI = 3.9 lesions/patient, FLAIR = 12.4 lesions/patient, CE-T1WI = 8.2 lesions/patient, DWI = 7.1 lesions/patient, and noncontrast T1WI = 3.4 lesions/patient. The average number of CT-hyperdense lesions was 0.4 lesions/patient. Most patients had regression of the brain lesions between 2–4 weeks (mean weeks: 2.5, range: 2–6) after the start of therapy (Fig. 6).
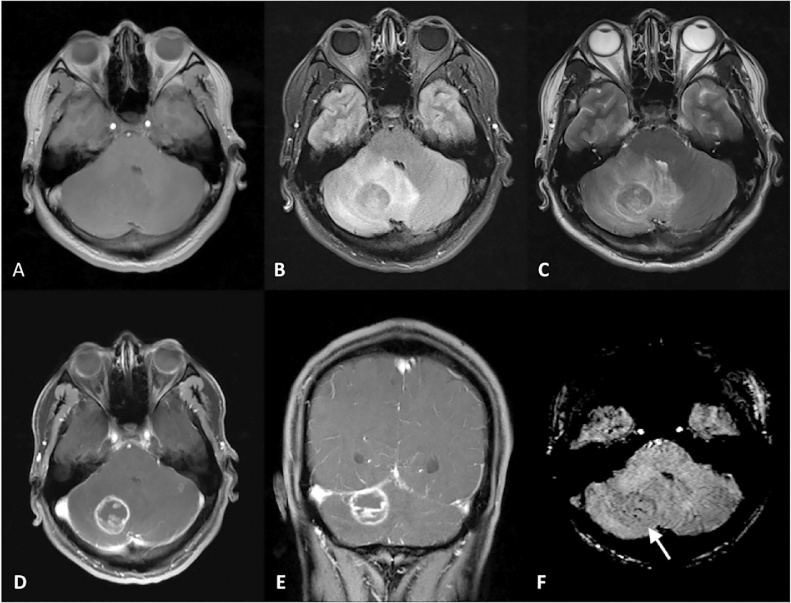
Fig. 1.
Noncontrast T1WI (A), FLAIR (B), T2WI (C), CE-T1WI axial (D) and coronal (E), and axial SWI (F) images in a 37 yearold patient with toxoplasmosis. A rim-enhancing lesion was noted in the right cerebellar hemisphere, with moderate associated surrounding edema noted on T2WI (B) and FLAIR (C). Several ISS were identified with the center of the lesion (white arrow on F). Other, less conspicuous SWI foci were also noted along the lesion’s periphery (not shown).
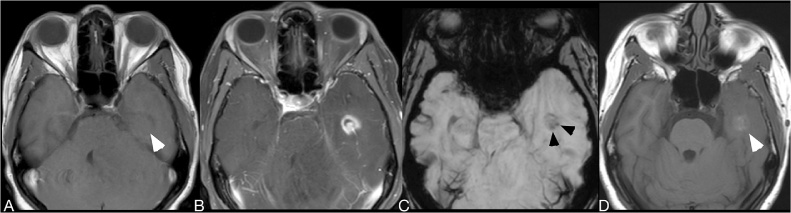
Fig. 2.
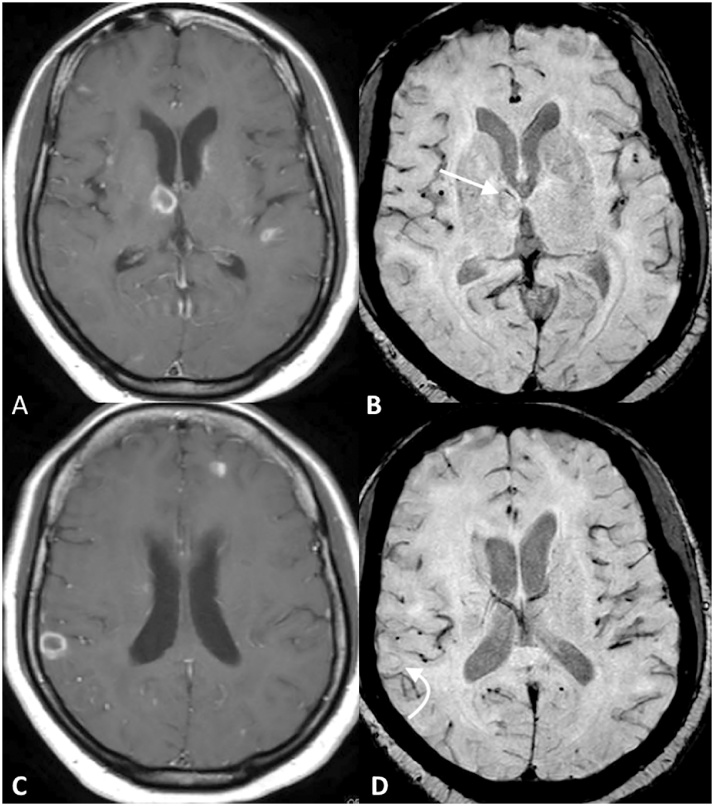
Baseline noncontrast T1WI (A), CE-T1WI (B), mIP SWI (C), and 2-week followup noncontrast T1WI images (D) of a patient treated with anti-toxoplasma medication. Two weeks later, the lesion in the left temporal lobe and a lesion in the left cerebellum (not shown) developed speckled intrinsic hyperintense foci on noncontrast T1WI (white arrowheads on A and D), a consequence that occurs in response to protein hydration layers that fill up the cytoplasm of reactive astrocytes. Two susceptibility signal foci (black arrowheads on D) are shown in the left temporal lobe lesion. While the SWI signal abnormalities remained stable on followup, the unusual T1-hyperintense signal changes was a transitory condition detected after empiric anti-toxoplasma therapy on most patients with toxoplasmosis.
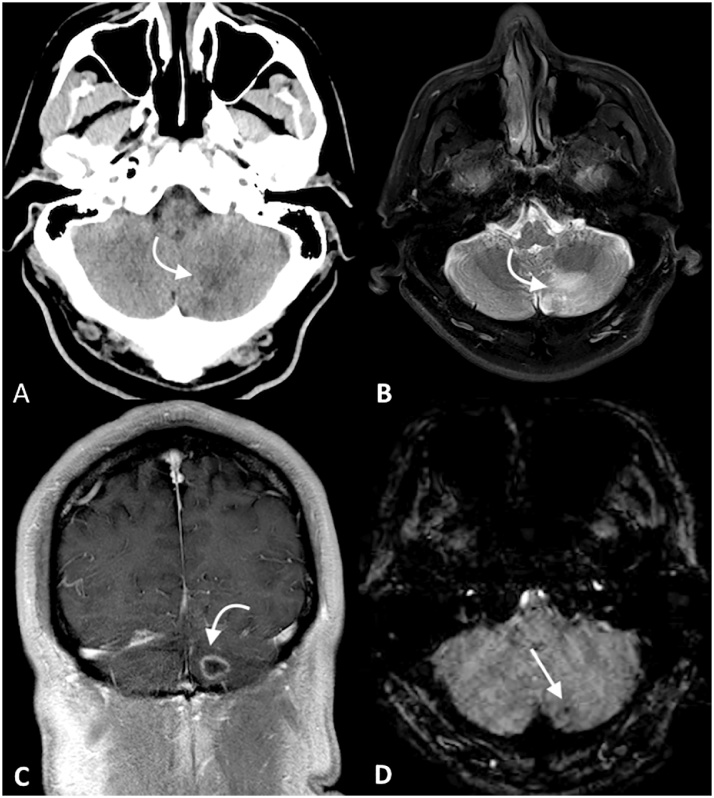
Fig. 3.
Patient who presented with alterered mental status and a recent syncopal event. NCCT (A) demonstrated an ill-defined low-density lesion in the left cerebellar hemisphere (curved arrow on A, B, and C), as well as other lesions within the left basal ganglia (not shown). MR demonstrated hyperintense signal within this region on T2WI (B). On coronal contrast-enhanced T1WI (C), a ring-enhancement was noted within this region; a single intralesional susceptibility signal focus was identified (D).
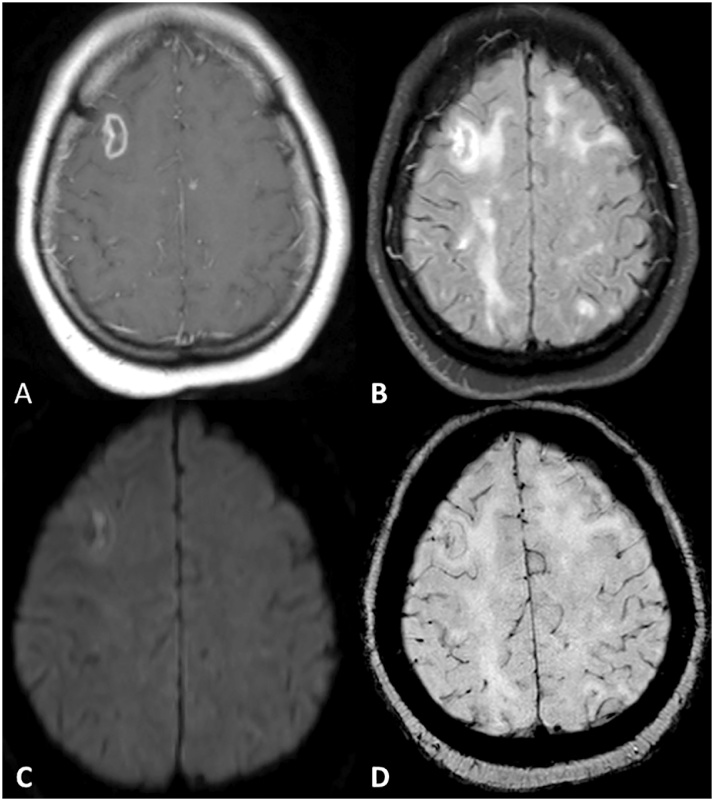
Fig. 4.
A patient with known toxoplamosis and poor adherence to medication presented with new neurologic symptoms. Contrast enhanced T1WI (A) showed a ring-enhancing lesion in the right frontal lobe. Mixed intensity within this region was noted on corresponding FLAIR (B) images, as well as other scattered white matter disease. The lesion demonstrated mild restricted diffusion (C) as well as intralesional susceptibility signal foci (D).
Fig. 5.
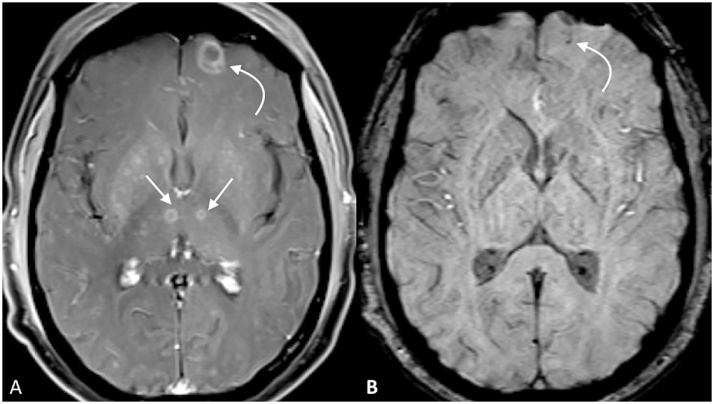
Multiple ring-enhancing lesions in a patient with toxoplasmosis are noted (A and C). Intralesional susceptibility signal foci are identified within each lesion: including the medial right thalamus (straight arrow, B) and the gray-white junction of the cerebral cortex (curved arrow, D).
Fig. 6.
Numerous ring enhancing lesions in a 39 year old patient with toxoplamosis. Many of the larger lesions (curved arrow, A) demonstrated intralesional susceptibility signal foci (curved arrow, B), while some of the smaller lesions did not (straight arrows, A).
On DWI, 13/15 (86.7%) patients had at least one bright lesion with reduced diffusion on ADC maps. By comparing noncontrast T1 signal abnormality on pre- and post-anti-toxoplasma therapy imaging, the presence of speckled T1-hyperintense foci during the followup MRI was evident in 9/15 (60%) of patients.
4. Discussion
The current study found that SWI abnormalities are present in the vast majority of patients with neurotoxoplasmosis. Of the other imaging sequences studied, abnormalities were most commonly identified on FLAIR, followed by CE-T1WI. Lesions were less likely to be identified on NCCT. Thus, SWI could be of utility in characterizing neurotoxoplasmosis and in differentiating this disorder from others in immunocompromised patients, such as primary CNS lymphoma, as the vast majority of primary CNS lymphoma lesions do not contain ISS on SWI [21]. Future, prospective studies should compare the frequency of ISS on SWI in various immunocompromised disorders of the CNS.
The capability of SWI to identify hemorrhagic lesions makes it a useful tool in characterizing hemorrhagic foci in neurotoxoplasmosis. It is superior to GRE T2*WI in determining the frequency, distribution, and size of hemorrhages, likely due to GRE T2*WI’s lack of postprocessing of the combined magnitude and phase data, as well as the inferior spatial-resolution [22,23]. The prior literature on microhemorrhagic foci in patients with toxoplasmosis is scant; one study found microhemorrhagic foci in a minority of patients on NCCT, while a more recent series found one or multiple hemorrhagic foci in 7 of 11 patients based on either CT or T1WI MRI [13,[24], [25], [26]]. Thus, the superior sensitivity of SWI in the detection of hemorrhage likely accounts for the greater frequency of observed foci noted in the current study.
The precise mechanism causing the observed hemorrhagic foci is unclear. However, focal or multifocal necrotizing abscesses are the histologic hallmark of toxoplasmosis with tachyzoites and bradyzoites lodging in areas of acute necrosis in T. gondii lesions [27]. Acutely infected necrotizing lesions in toxoplasmosis may produce damage to the walls of small arterioles, by progressing to focal destruction or thrombosis of small capillaries, arterioles and venules by parasitism or immune-mediated vasculitis, as previously described on histological specimens [24,27]. However, toxoplasmosis is not the only disorder in the immunocompromised that causes microhemorrhage. Vascular damage causing microhemorrhage can also occur in other diseases common in HIV/AIDS, including primary CNS lymphoma [25,28]. Further research is needed to assess whether ISS can be used to differentiate toxoplasmosis from these other processes.
To date, only a few studies have described the association of noncontrast T1-hyperintense signal changes in patients with neurotoxoplasmosis [13,14]. In this study’s population, speckled noncontrast T1-hyperintense signal on MR imaging was more readily and frequently identified. Such T1 signal abnormality seems not directly attributable to hemorrhage, as evidenced by the lack of associated ISS on SWI, or to calcification, given the lack of hyperdensity on CT. In addition, these changes appear to occur temporarily, as they mostly occurred between 2 and 4 weeks after the start of empiric therapy. Although technically indeterminate, it is possible that these foci represent a consequence of glial responses (gemistocytic astrocytosis).
Regarding the other foci observed, it should be noted that the frequency of abnormalities on each sequence does not imply specificity to neurotoxoplasmosis. For example, the current studied demonstrated greater frequency of FLAIR abnormalities than enhancing lesions. However, while the enhancing eccentric target sign is considered pathognomonic for neurotoxoplasmosis, FLAIR foci are relatively non-specific [12]. Hence, the frequency of abnormal foci in this study should be considered descriptive of neurotoxoplasmosis findings, rather than ordered in the list of specificity.
The study has several limitations. First, in the era of HAART therapy, the number of HIV-infected patients presenting with AIDS-defining opportunistic infections or neoplasms has decreased. Hence, a relatively small patient sample was available. Next, because it was difficult to count and compare the number of lesions on SWI if the stipulated maximum threshold of 20 FLAIR-positive lesions in this study was exceeded, the results are expressed as the average number of lesions. Because of this, there may have been slight underestimation of the overall number of lesions on SWI. In addition, because the maximum number of foci recorded was 20, comparisons between lesions (e.g. the proportion of enhancing foci that had ISS) were not available. Also, patients were imaged on two different MR scanners (1.5T and 3.0T), which may have affected the number of SWI foci observed. Next, because this study sought specifically to quantitate the frequency of ISS, while secondarily noting the number of abnormal foci on other sequences, the qualitative characteristics on each imaging sequence (e.g. the eccentric target sign) were not addressed; assessing the correlation between ISS and other imaging signs could be a subject of future studies. Finally, as noted above, hemorrhagic foci have also been described in primary CNS lymphoma. Although the presence of microhemorrhages may serve as a useful adjunct in the diagnosis of toxoplasmosis, further research is needed to determine if this imaging feature can be used to differentiate toxoplasmosis from primary CNS lymphoma and other disorders in the immunocompromised.
5. Conclusions
In our experience, abnormal SWI foci are present in the vast majority of patients with neurotoxoplasmosis, and are presumably attributable to hemorrhage. Although these findings appear to be common in toxoplasmosis, they are not specific to the process, and can also be seen in primary CNS lymphoma, which is an important diagnostic consideration. Future studies may be useful to determine if there is a difference in the frequency of SWI abnormalities between toxoplasmosis and CNS lymphoma. On other imaging sequences, abnormal foci were most commonly identified on FLAIR imaging, whereas CT abnormalities were rare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. None was utilized to produce the manuscript.
Conflict of interest
The authors of this study have no disclosures.
Footnotes
Institutional Review Board (IRB) approval was obtained for this retrospective study.
References
- 1.Buchacz K., Baker R.K., Palella F.J. IDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS. 2010;24:1549–1559. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 2.Castillo M. Brain infections in human immunodeficiency virus positive patients. Top. Magn. Reson. Imaging. 1994;6:3–10. [PubMed] [Google Scholar]
- 3.Ciricillo S.F., Rosenblum M.L. Use of CT and MR imaging to distinguish intracranial lesions and to define the need for biopsy in AIDS patients. J. Neurosurg. 1990;73:720–724. doi: 10.3171/jns.1990.73.5.0720. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz L.B., Hensley G.T., Chan J.C. The neuropathology of acquired immune deficiency syndrome. Arch. Pathol. Lab. Med. 1984;108:867–872. [PubMed] [Google Scholar]
- 5.Rosenblum M.L., Levy R.M., Bredesen D.E. Neurosurgical implications of the acquired immunodeficiency syndrome (AIDS) Clin. Neurosurg. 1988;34:419–445. [PubMed] [Google Scholar]
- 6.2015. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. Retrieved November 9 2015 from https://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. C1–2. [Google Scholar]
- 7.Antinori A. Evaluation and management of intracranial mass lesions in AIDS: report of the quality standards subcommittee of the american academy of neurology. Neurology. 1998;51:1233–1234. doi: 10.1212/wnl.51.4.1233. [DOI] [PubMed] [Google Scholar]
- 8.Berger J.R. Mass lesions of the brain in AIDS: the dilemmas of distinguishing toxoplasmosis from primary CNS lymphoma. Am. J. Neuroradiol. 2003;24:554–555. [PMC free article] [PubMed] [Google Scholar]
- 9.Navia B.A., Petito C.K., Gold J.W. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann. Neurol. 1986;19:224–238. doi: 10.1002/ana.410190303. [DOI] [PubMed] [Google Scholar]
- 10.Davaro R.E., Thirumalai A. Life-threatening complications of HIV infection. J. Intensive Care Med. 2007;22:73–81. doi: 10.1177/0885066606297964. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey R.G., Gean A.D. Neuroimaging of AIDS: I. Central nervous system toxoplasmosis. Neuroimaging Clin. N. Am. 1997;7:171–186. [PubMed] [Google Scholar]
- 12.Kumar G.G., Mahadevan A., Guruprasad A.S. Eccentric target sign in cerebral toxoplasmosis: neuropathological correlate to the imaging feature. JMRI. 2010;31:1469–1472. doi: 10.1002/jmri.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhagavati S., Choi J. Frequent hemorrhagic lesions in cerebral toxoplasmosis in AIDS patients. J. Neuroimaging. 2009;19:169–173. doi: 10.1111/j.1552-6569.2008.00274.x. [DOI] [PubMed] [Google Scholar]
- 14.Maeda T., Fujii T., Matsumura T. AIDS-related cerebral toxoplasmosis with hyperintense foci on T1-weighted MR images: a case report. J. Infect. 2006;53:167–170. doi: 10.1016/j.jinf.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Reichenbach J.R., Venkatesan R., Schillinger D.J. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204:272–277. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- 16.Haacke E.M., Mittal S., Wu Z. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. Am. J. Neuroradiol. 2009;30:19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haacke E.M., Xu Y., Cheng Y.-C.N. Susceptibility weighted imaging (SWI) Magn. Reson. Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 18.Rauscher A., Sedlacik J., Barth M. Magnetic susceptibility-weighted MR phase imaging of the human brain. AJNR Am. J. Neuroradiol. 2005;26:736–742. [PMC free article] [PubMed] [Google Scholar]
- 19.Haacke E.M., Ye Y. The role of susceptibility weighted imaging in functional MRI. Neuroimage. 2012;62:923–929. doi: 10.1016/j.neuroimage.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Mittal S., Wu Z., Neelavalli J. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. Am. J. Neuroradiol. 2009;30:232–252. doi: 10.3174/ajnr.A1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y., Xing Z., Liu B. Differentiation of primary central nervous system lymphoma from high-grade glioma and brain metastases using susceptibility-weighted imaging. Brain Behav. 2014;4:841–849. doi: 10.1002/brb3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong K.A., Ashwal S., Holshouser B.A. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology. 2003;227:332–339. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- 23.Haacke E.M., DelProposto Z.S., Chaturvedi S. Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. Am. J. Neuroradiol. 2007;28:316–317. [PMC free article] [PubMed] [Google Scholar]
- 24.Trenkwalder P., Trenkwalder C., Feiden W. Toxoplasmosis with early intracerebral hemorrhage in a patient with the acquired immunodeficiency syndrome. Neurology. 1992;42:436–438. doi: 10.1212/wnl.42.2.436. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhari A.B., Singh A., Jindal S. Haemorrhage in cerebral toxoplasmosis. A report on a patient with the acquired immunodeficiency syndrome. S. Afr. Med. J. 1989;76:272–274. [PubMed] [Google Scholar]
- 26.Revel M.P., Gray F., Brugieres P. Hyperdense CT foci in treated AIDS toxoplasmosis encephalitis: MR and pathologic correlation. J. Comput. Assist. Tomogr. 1992;16:372–375. doi: 10.1097/00004728-199205000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y.Y., Bruner J.M., Van Tassel P. Primary central nervous system lymphoma: CT and pathologic correlation. Am. J. Roentgenol. 1986;147:747–752. doi: 10.2214/ajr.147.4.747. [DOI] [PubMed] [Google Scholar]
- 28.Chang L., Cornford M.E., Chiang F.L. Radiologic-pathologic correlation: cerebral toxoplasmosis and lymphoma in AIDS. Am. J. Neuroradiol. 1995;16:1653–1663. [PMC free article] [PubMed] [Google Scholar]