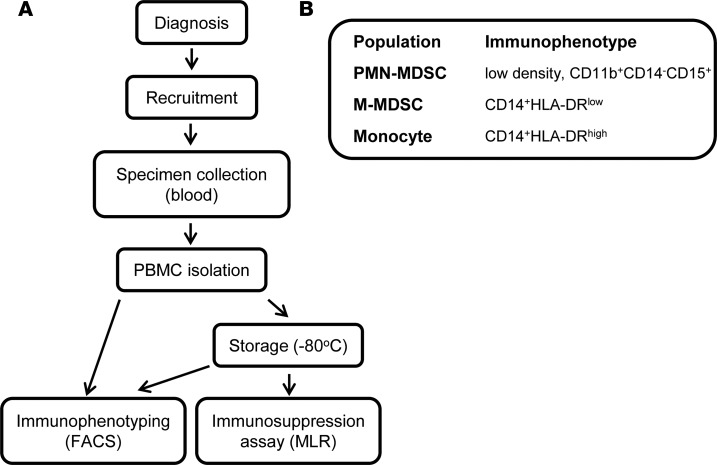
Figure 1. Study design.
(A) Patients diagnosed with stage I–IV CRC were recruited to the study after providing written informed consent. Blood samples were collected from the patients, and the PBMC fraction was separated for immunophenotyping or cryopreserved for future use in the immunosuppression assay and immunophenotyping. (B) Markers used to define myeloid cell subpopulations.