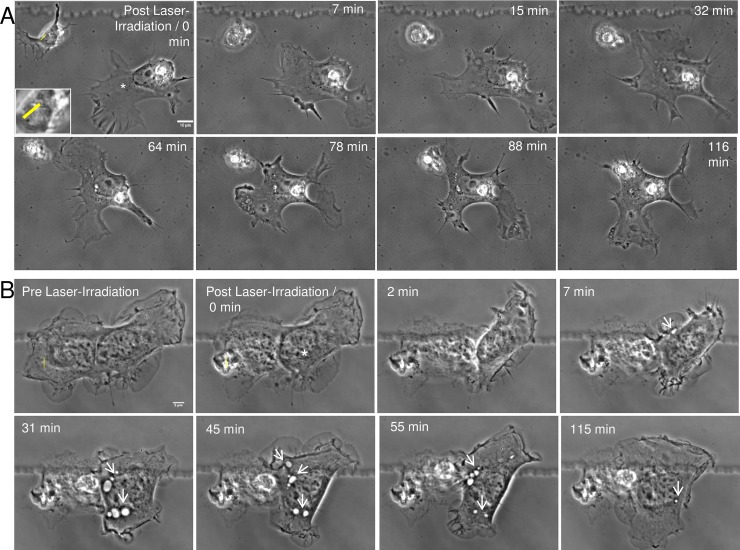
Fig 2. Neighboring astrocyte initiates phagocytosis of irradiated damaged/dead cell.
A. One of two single, isolated astrocytes (not sharing a membrane connection) from a cortical culture is lysed via laser nanosurgery. The “Post Laser-Irradiation / 0 min” image shows the two astrocytes immediately following laser exposure. The laser exposure site is denoted by a yellow ROI (magnified image of laser-targeted region visible in inset). Seven minutes following laser irradiation, necrosis of the targeted astrocyte is observed. The responding astrocyte (white asterisk) approaches the lysed cell material at 7 to 15 minutes, and by 32 minutes establishes a membrane connection. In the 0 minute image, the leading edge of the responding astrocyte is oriented toward the bottom of the image. Following laser irradiation, the responding astrocyte retracts its leading edge and subsequently migrates toward the necrotic laser-irradiated cell. Prior to and immediately following laser irradiation, there are no obvious vesicles in the cytoplasm of the leading edge of the responding astrocyte. However, seven minutes following laser irradiation, vesicles begin to form at the cell periphery and are observed migrating toward the nucleus. By 116 minutes, the responding astrocyte engulfs the necrotic material via phagocytosis. B. A representative astrocyte type-I (Ast-1) cell from an immortalized cell line responded to laser irradiation of a neighboring astrocyte (yellow ROI). Prior to laser exposure, the responding astrocyte (white asterisk) shared a membrane connection to the laser irradiated cell. Phagocytosis of the irradiated/killed cell was initiated seven minutes following laser exposure. An increase in membrane ruffling and extension toward the damaged cell or its debris was observed. Endocytic vesicles moved from the cell periphery toward the nucleus (white arrowheads). Vesicles increased in size and number over the initial 45 minutes, and appeared to break down 55–115 minutes following laser irradiation.