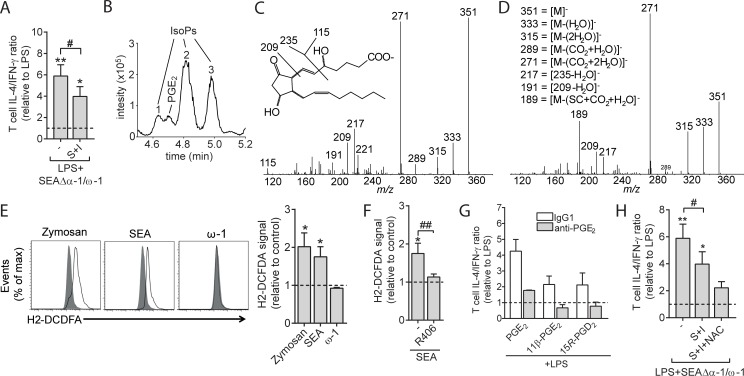
Fig 5. SEA-induced ROS production by moDCs results in PGE2 isomer synthesis that contributes to Th2 polarization.
(A) T-cell polarization assay as described in Fig 2A in the presence of COX inhibitors S and I. Bars represent mean ± SEM of at least 3 independent experiments. (B) LC-MS/MS trace showing the transition m/z 351 → 271; the detected IsoPs are indicated by numbers. (C) Tandem MS spectrum of isomer 2, showing the fragment ion m/z 189, characteristic for 15-series IsoPs. (D) Showing the MS/MS spectrum of isomer 3, possibly identifying this isomer as a 5-series IsoP, based on the fragment ions m/z 115, 217, and 191. (E) ROS generation was determined by flow cytometry (H2-DCFDA) of moDCs pulsed for 6 h with indicated reagents. On the left, representative histograms for ROS induction are shown. On the right, the geometric mean fluorescence of these signals is enumerated and shown as fold change relative to unstimulated moDCs (dashed line set to 1). (F) ROS production was quantified as in panel E following pretreatment with general ROS scavenger NAC or R406 for 1 h. (G) moDCs were stimulated with indicated PGs with or without anti-PGE2 after which a T-cell polarization assay was performed as described in Fig 2A. (H) moDCs were stimulated with indicated reagents in the presence following 1 h pre-incubation with COX inhibitors (S and I) and NAC after which a T-cell polarization assay was performed as described in Fig 2A. Bars represent mean ± SEM of at least 3 independent experiments. “*” and “#”: P < 0.05: “**” and “#”: P < 0.01 for significant differences with the LPS control (*) or between-test conditions (#) based on paired analysis (paired Student t test). Underlying data can be found in S1 Data. COX, cyclooxygenase; H2-DCFDA, 2',7'-dichlorodihydrofluorescein diacetate; I, indomethacin; IsoP, isoprostane; LC-MS/MS, liquid chromatography tandem mass spectrometry; LPS, lipopolysaccharide; moDC, monocyte-derived DC; NAC, N-acetyl-L-cysteine; PGE2, prostaglandin E2; ROS, reactive oxygen species; S, SC236; SEA, soluble egg antigen; Th2, T helper 2.