Abstract
BACKGROUND:
Myeloperoxidase (MPO) is an enzyme involved in the pathogenesis of several diseases.
AIM:
The current study aimed to investigate serum MPO levels in obese Egyptian women and assess its relation with insulin resistance (IR) and other biochemical risk parameters.
METHODS:
The study included 80 obese women and 50 age-and-sex-matched healthy controls. Insulin resistance (IR) was evaluated by the Homeostasis Model Assessment-Insulin Resistance (HOMA-IR). Serum MPO, fasting glucose, insulin and blood lipids and anthropometry were measured. Obese cases were divided into three groups based on MPO tertiles. ROC analysis was performed to obtain the optimal cut-off values of MPO to predicate IR in obese women.
RESULTS:
The mean serum MPO was significantly higher in obese cases than controls. Cases in the highest MPO tertile had higher HOMA-IR, blood lipids and pressure levels compared with those in the lower tertile. The cutoff point of MPO was > 87.8 (ng/mL) and area under curves was 0.82 (p < 0.01) for diagnosis of IR. MPO levels were higher in obese Egyptian women than healthy controls.
CONCLUSION:
Elevation of MPO was associated with abnormal metabolic parameters. MPO might be used as an earlier biomarker for IR and metabolic disturbance in obese women.
Keywords: Keywords Myeloperoxidase, Insulin resistance, Blood lipids, Obesity, Women
Introduction
MPO is a heme peroxidase that is highly expressed in leukocytes and considered as a principal enzyme in the innate immune response. It is substantially stored in cytoplasmic granules and might be discharged into the extracellular compartment following phagocyte activation [1]. As MPO can commence lipid peroxidation process, it acts as an earlier marker of oxidative damage [2]. In this regard, lots of evidence indicate the association between the oxidative stress and metabolic syndrome (MS) parameters [3] [4], where oxidative stress affects the pathophysiology of MS and its components [5]. Although MPO plays an important role in the innate immune system, it has a very deleterious effect on a large number of inflammatory-mediated diseases. It has been found in cases with an acute coronary syndrome that the serum level of MPO is highly correlated with elevated risk of subsequent cardiovascular diseases.
Central obesity, dyslipidemia, insulin resistance, and hypertension constitute the main components of the metabolic syndrome and metabolically linked to cardiovascular risk factors [6]. Levels of antioxidants in metabolic syndrome cases varied in different races [7]. Plasma levels of MPO are found to be high in patients with stable coronary artery disease. Furthermore, it has been demonstrated that MPO is a powerful prognosticator of the undesirable clinical outcomes in cases with chronic heart failure, acute coronary syndrome, and of future coronary artery disease in a healthy population. Moreover, the main risk factor for cardiovascular diseases and mortality, type 2 diabetes, is positively correlated with MPO levels [8]. It seems that the MPO plays a role as a mediator in the vascular inflammation and in the production of oxidant species that are important in the pathophysiology of the inflammatory diseases. Therefore, this study aimed to evaluate the association between serum levels of MPO with metabolic and biochemical parameters in obese women.
Methods
The study sample included 80 obese women and 50 age-and-sex-matched healthy controls. Obese cases were divided into three groups according to MPO tertiles. This research has been approved by the Ethical Committee of National Research Centre, Egypt (number = 16361), by the World Medical Association’s Declaration of Helsinki.
All patients and controls were subjected to full medical history and clinical examination. All anthropometric measurements were taken 3 times on the left side of the body, and the mean of the 3 values was used. Body weight was measured to the nearest 0.1 kg and height was measured to the nearest 0.1 cm. Height was measured with the patients standing with their backs leaning against a stadiometer scale. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Waist circumference (WC) and hip circumference (HC) were measured in cm using a plastic, non-stretchable tailor’s tape. WC was measured with light clothing at a level midway between the lower rib margin and the iliac crest standing and breathing normally. HC was measured at the level at the widest circumference over the buttocks (at the greater trochanter). Subsequently, the waist-hip ratio (WHR) was calculated as WC divided by HC. Skin-fold thickness was measured to the nearest mm, except for low values (usually 5 mm or less) where it was taken to the nearest 0.5 mm. These readings were done at the biceps, triceps, subscapular, supra-iliac and abdominal areas using Holtain calliper. The biceps skin-fold thickness was measured at the level of the mid-point between the acromion (lateral edge of the acromion process) and the radius (proximal and lateral border of the radius bone) on the mid-line of the anterior surface of the arm. Triceps skin-fold thickness was measured vertically at the midway between acromion and olecranon processes on the posterior surface of the arm. The subscapular skin-fold was measured below the lower angle of the left scapula at a diagonal in the natural cleavage of the skin. The position of the suprailiac skinfold was the diagonal fold just above the iliac crest even with the anterior axillary line. Abdominal skin-fold was at 5 cm adjacent to the umbilicus to the right side. Subsequently, the sum of skinfolds was calculated. Anthropometric measurements were obtained according to standardised equipment and following the recommendations of the International Biological Program [9]. Body fat % was assessed by Tanita Body Composition Analyzer (SC -330).
Presence of IR was defined by HOMA-IR > 2.47 [10].
Systolic and diastolic blood pressures (SBP and DBP) have been measured twice in the right arm in a sitting position after a 10-min rest period, and the average of the two measurements was used for analysis. Blood pressure was measured according to a standardised operating procedure using a calibrated sphygmomanometer and brachial inflation cuff (HEM -7200 M3, Omron Healthcare, Kyoto, Japan).
Venous blood samples were collected by direct venipuncture after an overnight fast (minimum 12 h). Fasting plasma glucose and serum lipids (total cholesterol, high-density lipoprotein cholesterol (HDL-C) triglycerides (TG) were measured by enzymatic colourimetric methods using a Hitachi autoanalyser 704 (Roche Diagnostics. Switzerland). Low-density lipoprotein cholesterol (LDL-C) was calculated according to certain equation (LDL – C = Total cholesterol-Triglycerides/5 + HDL-C). Serum insulin concentration was analysed by chemiluminescent immunoassay (Immulite2000, Siemens, Germany. Insulin resistance was determined by the Homeostasis Model Insulin Resistance (HOMA-IR) is calculated as the product of the fasting plasma insulin level (IU/mL) and the fasting plasma glucose level (mmol/L), divided by 22.5 [11]. Clinical history and physical examination were performed for each subject.
Serum myeloperoxidase (MPO) was estimated using Quantikine ELIZA kit of R&D systems catalogue number DMYE00B for the quantitative determination of human MPO concentration in serum according to the manufacturer’s instructions.
All statistical analyses were performed using SPSS16.0 for Windows (SPSS Inc). The Kolmogorov-Smirnov test of normality was used to verify whether the distribution of variables followed a Gaussian pattern. Normally distributed data in groups were expressed as means ± SDs. A receiver operating characteristic (ROC) curve analysis was performed to obtain the optimal cutoff values of MPO to diagnosis IR. The optimal cutoff values were obtained both from the point on the ROC curve. The area under the curve (AUC) and the 95% confidence interval (CI) were used for diagnostic validity. The Youden index, calculated as (sensitivity + specificity-1) was estimated to determine optimal cut-off.
Differences in clinical and biochemical characteristics between groups were tested using one-way analysis of variance (ANOVA) and post hoc tests for differences between groups.
Results
Table 1 shows the clinical and biochemical characteristics of obese cases and controls. Mean age of obese women participated in the study was 31.5 ± 4.8 years and was 32.7 ± 4.7 in controls. Obese women showed significantly higher values of BMI, MPO, waist circumference, SBP, DBP, FBG, total cholesterol, TG, LDL-C and lower HDL-C than controls.
Table 1.
Clinical and biochemical characteristics of obese cases and controls
| Characteristics | Obese | Controls |
|---|---|---|
| Age (years) | 31.5 ± 4.8 | 32.7 ± 4.7 |
| BMI (kg/m2) | 32.21 ± 5.4* | 22.24 ± 4.2 |
| WC (cm) | 96.2 ± 6.12* | 81.6 ± 4.3 |
| WHR | 0.82 ± 0.8 | 0.79 ± 0.6 |
| Sum SF | 149. 9 ± 23. 9 | 143.9 ± 21.9 |
| Body fat% | 29.9 ± 9.9 | 27.5 ± 8.4 |
| SBP (mmHg) | 135.55 ± 9.8* | 100. 3 ± 9.4 |
| DBP (mmHg) | 88.8 ± 6.9* | 65.24 ± 8.8 |
| HOMA-IR | 6.8 ± 1.2* | 2.2 ± 1.2 |
| FBG (mg/dL) | 111.4 ± 24.7* | 81. 0 ± 21.1 |
| TC (mg/dL) | 189.9 ± 36.2* | 118.9 ± 22.2 |
| TG (mg/dL) | 146.8 (120-148.8) * | 47.55 ± 10.45 |
| HDL-C (mg/dL) | 35.6 ± 4.9* | 47.77 ± 10.8 |
| LDL-C (mg/dL) | 163 (88-162)* | 165 (88-164)* |
| MPO (ng/mL ) | 86 (66 – 92)** | 22 ± 12 (20–30) |
BMI: body mass index; WC: waist circumference; WHR: waist to hip ratio; Sum SF: sum of skin folds; SBP: systolic blood pressure; DBP: diastolic blood pressure; HOMA-IR: homeostasis model assessment-insulin resistance; FBG: fasting glucose; TC: total cholesterol; TG: triglycerides; HDL -C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; MPO: myeloperoxidase;
p < 0.05,
p < 0.001.
Biochemical and metabolic characteristics in obese women according to the different levels of MPO tertiles are summarised in Table 2. Compared with participants in the first tertile of MPO, those in the third tertile had higher levels of HOMA-IR, total cholesterol, TG, LDL-C, BP and lower levels of HDL-C. Fig 1 shows ROC curves for MPO to identify IR in obese women, the cutoff points for MPO was > 87.8 (ng/mL) and area under curves was 0.82 (sensitivity 93.7%, specificity 71.4%) as a most sensitive/specific cut off.
Table 2.
Biochemical and metabolic characteristics in obese women according to the Myeloperoxidase (MPO) tertiles
| Characteristic | I Lower tertile ≤ 61.7 | II Intermediate tertile 61.8-82.6 | III Higher tertile > 82.7 |
|---|---|---|---|
| Age (years) | 31.2±4.1 | 33.5±3.5 | 31.1 ±4.8 |
| BMI(kg/m2) | 31.8 ± 5.4 | 33.4 ± 6.6 | 34.9 ± 6.23 |
| WC (cm) | 86.0 ± 10.9 | 87.9 ± 11.1 | 96.9 ± 10.9* |
| WHR | 0.7 ± .8 | 0.8 ± .7 | 0.9 ± .9 |
| Sum SF | 131.6 ±10.5 | 134.2 ± 11.0 | 138.4 ± 14.9 |
| Body fat% | 29.9 ± 9.8 | 32.6 ± 10.9 | 33.5 ± 12.8 |
| SBP (mmHg) | 111.1±10.5 | 125.4 ± 9.4 | 151.3 ± 11.4* |
| DBP (mmHg) | 71.5 ± 8.6 | 76.3±6.8 | 89.9±8.5* |
| HOMA-IR | 3.5 ± 1.1 | 3.7 ± .7 | 5.6 ± 1.2* |
| FBG (mg/dL) | 83.1 ± 20.8 | 86.3 ± 25.7 | 121. 8 ± 22.9* |
| TC (mg/dL) | 136.7 ± 20.7 | 149.9 ± 21.9 | 193 ± 23.5* |
| TG (mg/dL) | 139.9 ± 20.2 | 149.8 ± 22.5 | 195.8 ± 21.5* |
| HDL-C (mg/dL) | 46.4 ± 10.5 | 41. 6 ± 11.4 | 35.4±12.2* |
| LDL-C (mg/dL) | 124.7 ± 21.3 | 129.3 ± 20.9 | 156.6 ± 21.9* |
| MPO (µg/L) | 54.1 (33.4-57.9) | 69 (60.7-66.9) | 91.3 (83.4- 96.9) * |
BMI: body mass index; WC: waist circumference; WHR: waist to hip ratio; Sum SF: sum of skin folds; SBP: systolic blood pressure; DBP: diastolic blood pressure; HOMA-IR: homeostasis model assessment-insulin resistance; FBG: fasting glucose; TC: total cholesterol; TG: triglycerides; HDL -C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; MPO: myeloperoxidase; Data are mean ± SD, median (intertertile range); Tertile I vs Tertile III
p < 0.05.
Figure 1.
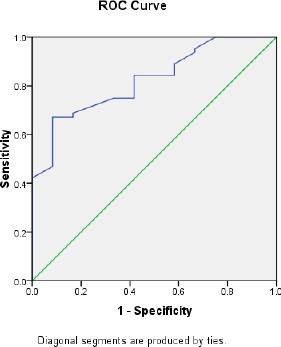
the Receiver-operating characteristic curve of sensitivity plotted against1-specificity of serum MPO to identify IR among obese women
Discussion
This study showed that MPO is positively associated with IR in the obese women. As it is linked to obesity, IR is kept rising and consequently Type 2 Diabetes affects a large number of young individuals [8]. It has been demonstrated that elevated concentrations of glucose and free fatty acids lead to oxidative stress and start IR in genetically predisposed individuals with diabetes [12]. Previous studies have found that MPO might be used as a predictor of both early [13] [14] and late unfavourable cardiac events in cases suffered from chest pain and other clinical features of the acute coronary syndrome. MPO plays an important role in the oxidation of HDL in vivo [14] which result in functional inactivation. The subsequent decrease in plasma HDL causes a reduction in the reverse cholesterol transport which stimulates atherosclerosis [15]. Moreover, HDL is a very important anti-inflammatory, antioxidant, and antithrombotic factor. In patients with metabolic syndrome, the activated leukocytes might be responsible for the increased activity of MPO. Furthermore, insulin resistance causes higher concentrations of proinflammatory mediators [16]. Also, the inflammatory cytokines are produced by adipocytes, especially in obese individuals, to compromise the insulin signalling [17]. In MS patients, both insulin resistance and central obesity are demonstrated, however, the association between the augmented MPO expression and chronic inflammation is also evident [18]. The connection between proinflammatory regulators and pathogenesis of the MS has been previously demonstrated, where the impairment in the inflammatory response could be noticed during the disease [19]. MPO acts as a physiological mechanism that connects the matrix proteins degradation by metalloproteinases.
Our results found a higher concentration of MPO in obese group than controls. Also, we found that cases in the higher tertile of MPO had higher levels of HOMA-IR, total cholesterol, TG, LDL-C, SBP, DBP and lower levels of HDL cholesterol than those in the lower tertile. The present analysis showed that the cutoff point of MPO was > 87.8 (ng/mL) for diagnosis of IR in obese Egyptian women (p < 0.01). In agreement with our findings, it has been previously reported that MPO is associated with IR [20]. It is evident that MPO is an effective mediator of endothelial dysfunction where a strong connection has been observed between serum MPO levels and endothelial dysfunction in overweight subjects [21]. Obesity is the principal cause for low HDL, and in order to enhance the HDL level, an optimizing healthy lifestyle is required including moderate weight loss along with exercise and smoking cessation. High oxidative stress and systemic inflammation are correlated with increased plasma triglyceride-rich lipoproteins and oxidized lipoprotein (a) phospholipids which lead to cardiovascular risks [22] [23]. Losing of the anti-oxidative, anti-inflammatory and athero-protective properties of HDL and its apolipoproteins could even increase those biochemical disturbances. Metabolic disorders such as insulin resistance, oxidative stress, inflammation, and hyperlipidemia represent connected disturbances in women with metabolic syndrome [24]. Obesity has certain anthropometric, biochemical and physiological anomalies that lead to IR and cardiovascular disease (CVD). Therefore, numerous studies have indicated the associations between obesity, inflammation, CVD and IR in both adults and children [25]. MPO activity was used as an adjuvant marker for the inflammatory and oxidative status in obese women [26] [27] [28]. Also, there is increasing evidence that MPO contributes to cardiovascular disease. It has been reported that the presence of obesity in childhood stage in association with the risk factors hurt the vascular health status in adulthood [29]. Furthermore, in apparently healthy adults, augmented serum MPO levels were found to be associated with higher CVD risk [30].
In conclusion, MPO might be used as an earlier biomarker for IR in obese women and might be useful in determining patients who are at high risk for a biochemical disturbance.
Footnotes
Funding: This study was supported by a grant from National Research Centre, Egypt.
Competing Interests: The authors have declared that no competing interests exist
References
- 1.von Leitner E-C, Klinke A, Atzler D, Slocum JL, Lund N, Kielstein JT, et al. Pathogenic cycle between the endogenous nitric oxide synthase inhibitor asymmetrical dimethylarginine and the leukocyte-derived hemoprotein myeloperoxidase. Circulation. 2011;124(24):2735–45. doi: 10.1161/CIRCULATIONAHA.111.060541. https://doi.org/10.1161/CIRCULATIONAHA.111.060541. PMid:22082678. [DOI] [PubMed] [Google Scholar]
- 2.Zhang R, Brennan M-L, Shen Z, MacPherson JC, Schmitt D, Molenda CE, et al. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem. 2002;277(48):46116–22. doi: 10.1074/jbc.M209124200. https://doi.org/10.1074/jbc.M209124200. PMid:12359714. [DOI] [PubMed] [Google Scholar]
- 3.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40(2):295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. https://doi.org/10.1016/j.freeradbiomed.2005.08.025. PMid:16413411. [DOI] [PubMed] [Google Scholar]
- 4.Yubero-Serrano EM, Delgado-Lista J, Pena-Orihuela P, Perez-Martinez P, Fuentes F, Marin C, et al. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Exp Mol Med. 2013;45(6):e28. doi: 10.1038/emm.2013.53. https://doi.org/10.1038/emm.2013.53. PMid:23788131. PMCid: PMC3701288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations. Diabetes. 2003;52(9):2346–52. doi: 10.2337/diabetes.52.9.2346. https://doi.org/10.2337/diabetes.52.9.2346. PMid:12941775. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome. Circulation. 2004;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. https://doi.org/10.1161/01.CIR.0000111245.75752.C6. PMid:14744958. [DOI] [PubMed] [Google Scholar]
- 7.Morris AA, Zhao L, Patel RS, Jones DP, Ahmed Y, Stoyanova N, et al. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) study. Metab Syndr Relat Disord. 2012;10(4):252–9. doi: 10.1089/met.2011.0117. https://doi.org/10.1089/met.2011.0117. PMid:22385338. PMCid: PMC3449394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiersma JJ, Meuwese MC, van Miert JNI, Kastelein A, Tijssen JGP, Piek JJ, et al. Diabetes mellitus type 2 is associated with higher levels of myeloperoxidase. Med Sci Monit. 2008;14(8):CR406–10. PMid:18667997. [PubMed] [Google Scholar]
- 9.Hiernaux J, Tanner JM, Jarman S. Growth and physical studies. Hum Biol A Guid to F methods London IBP. 1969 [Google Scholar]
- 10.Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, Gude F, García F, De Francisco A, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013;13(1):47. doi: 10.1186/1472-6823-13-47. https://doi.org/10.1186/1472-6823-13-47. PMid:24131857. PMCid: PMC4016563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. https://doi.org/10.1007/BF00280883. PMid:3899825. [DOI] [PubMed] [Google Scholar]
- 12.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23(5):599–622. doi: 10.1210/er.2001-0039. https://doi.org/10.1210/er.2001-0039. PMid:12372842. [DOI] [PubMed] [Google Scholar]
- 13.Brennan M-L, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349(17):1595–604. doi: 10.1056/NEJMoa035003. https://doi.org/10.1056/NEJMoa035003. PMid:14573731. [DOI] [PubMed] [Google Scholar]
- 14.Cavusoglu E, Ruwende C, Eng C, Chopra V, Yanamadala S, Clark LT, et al. Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am J Cardiol. 2007;99(10):1364–8. doi: 10.1016/j.amjcard.2006.12.060. https://doi.org/10.1016/j.amjcard.2006.12.060. PMid:17493461. [DOI] [PubMed] [Google Scholar]
- 15.Marsche G, Hammer A, Oskolkova O, Kozarsky KF, Sattler W, Malle E. Hypochlorite-modified high density lipoprotein, a high affinity ligand to scavenger receptor class B, type I, impairs high density lipoprotein-dependent selective lipid uptake and reverse cholesterol transport. J Biol Chem. 2002;277(35):32172–9. doi: 10.1074/jbc.M200503200. https://doi.org/10.1074/jbc.M200503200. PMid:12070141. [DOI] [PubMed] [Google Scholar]
- 16.Kim JA, Choi YS, Hong JI, Kim SH, Jung HH, Kim SM. Association of metabolic syndrome with white blood cell subtype and red blood cells. Endocr J. 2006;53(1):133–9. doi: 10.1507/endocrj.53.133. https://doi.org/10.1507/endocrj.53.133. PMid:16543683. [DOI] [PubMed] [Google Scholar]
- 17.Babio N, Ibarrola-Jurado N, Bulló M, Martínez-González MÁ, Wärnberg J, Salaverría I, et al. White blood cell counts as risk markers of developing metabolic syndrome and its components in the PREDIMED study. PLoS One. 2013;8(3):e58354. doi: 10.1371/journal.pone.0058354. https://doi.org/10.1371/journal.pone.0058354. PMid:23526980. PMCid: PMC3602299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galijasevic S, Saed GM, Diamond MP, Abu-Soud HM. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc Natl Acad Sci. 2003;100(25):14766–71. doi: 10.1073/pnas.2435008100. https://doi.org/10.1073/pnas.2435008100. PMid:14657339. PMCid: PMC299800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Wang D, Li D, Sun R, Xia M. Associations of retinol-binding protein 4 with oxidative stress, inflammatory markers, and metabolic syndrome in a middle-aged and elderly Chinese population. Diabetol Metab Syndr. 2014;6(1):25. doi: 10.1186/1758-5996-6-25. https://doi.org/10.1186/1758-5996-6-25. PMid:24559154. PMCid: PMC3938900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pignatelli P, Loffredo L, Martino F, Catasca E, Carnevale R, Zanoni C, et al. Myeloperoxidase overexpression in children with hypercholesterolemia. Atherosclerosis. 2009;205(1):239–43. doi: 10.1016/j.atherosclerosis.2008.10.025. https://doi.org/10.1016/j.atherosclerosis.2008.10.025. PMid:19081093. [DOI] [PubMed] [Google Scholar]
- 21.Vita JA, Brennan M-L, Gokce N, Mann SA, Goormastic M, Shishehbor MH, et al. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110(9):1134–9. doi: 10.1161/01.CIR.0000140262.20831.8F. https://doi.org/10.1161/01.CIR.0000140262.20831.8F. PMid:15326065. PMCid: PMC2718053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Rasadi K, Al-Zakwani I, Zubaid M, Ali A, Bahnacy Y, Sulaiman K, et al. Prevalence, predictors, and impact of low high-density lipoprotein cholesterol on in-hospital outcomes among acute coronary syndrome patients in the Middle East. Open Cardiovasc Med J. 2011;5(1) doi: 10.2174/1874192401105010203. https://doi.org/10.2174/1874192401105010203. PMid:21966331. PMCid: PMC3178900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onat A, Can G, Yüksel H. Dysfunction of high-density lipoprotein and its apolipoproteins: new mechanisms underlying cardiometabolic risk in the population at large. Turk Kardiyol Dern Ars. 2012;40(4):368–85. doi: 10.5543/tkda.2012.55490. https://doi.org/10.5543/tkda.2012.55490. PMid:22951857. [DOI] [PubMed] [Google Scholar]
- 24.Feng RN, Niu YC, Sun XW, Li Q, Zhao C, Wang C, et al. Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2013;56(5):985–94. doi: 10.1007/s00125-013-2839-7. https://doi.org/10.1007/s00125-013-2839-7. PMid:23361591. [DOI] [PubMed] [Google Scholar]
- 25.Daniels SR. Complications of obesity in children and adolescents. Int J Obes. 2009;33(S1):S60. doi: 10.1038/ijo.2009.20. https://doi.org/10.1038/ijo.2009.20. PMid:19363511. [DOI] [PubMed] [Google Scholar]
- 26.Koh Y, Park J, Carter R. Oxidized Low-Density Lipoprotein and Cell Adhesion Molecules Following Exercise Training. Int J Sports Med. 2017;2017 doi: 10.1055/s-0043-118848. PMid:29190851. [DOI] [PubMed] [Google Scholar]
- 27.Rocha-Penha L, Bettiol H, Barbieri MA, Cardoso VC, Cavalli RC, Sandrim VC. Myeloperoxidase is not a good biomarker for preeclampsia prediction. Sci Rep. 2017;7(1):10257. doi: 10.1038/s41598-017-09272-4. https://doi.org/10.1038/s41598-017-09272-4. PMid:28860607. PMCid: PMC5579011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gariballa S, Alkaabi J, Yasin J, Al Essa A. Oxidative damage and associated inflammatory risk factors in obese Emirati women: Body mass index versus waist circumference. Saudi Med J. 2017;38(9):960. doi: 10.15537/smj.2017.9.19629. https://doi.org/10.15537/smj.2017.9.19629. PMid:28889156. PMCid: PMC5654032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman DS, Dietz WH, Tang R, Mensah GA, Bond MG, Urbina EM, et al. The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa Heart Study. Int J Obes. 2004;28(1):159. doi: 10.1038/sj.ijo.0802515. https://doi.org/10.1038/sj.ijo.0802515. PMid:14581934. [DOI] [PubMed] [Google Scholar]
- 30.Meuwese MC, Stroes ESG, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;50(2):159–65. doi: 10.1016/j.jacc.2007.03.033. https://doi.org/10.1016/j.jacc.2007.03.033. PMid:17616301. [DOI] [PubMed] [Google Scholar]


