Abstract
Purpose
Cardiovascular magnetic resonance imaging exams can be performed during free-breathing. This may be especially important for boys with Duchenne muscular dystrophy (DMD) given their frequently limited breath-hold abilities. The impact of the respiratory compensation method on quantitative measurements of left ventricular (LV) rotational mechanics is incompletely understood. The purpose of this study was to evaluate differences in LV rotational mechanics acquired during breath-holding (BH), free-breathing with averaging (AVG), and free-breathing with respiratory bellows gating (BEL).
Methods
LV short-axis tagged images from healthy subjects (N=16) and DMD patients (N=5) were acquired with BH, AVG, and BEL. LV twist and circumferential-longitudinal shear (CL-shear) angle were measured using the Fourier Analysis of STimulated echoes (FAST) method.
Results
Peak LV twist estimates using BEL were significantly lower compared with BH in both healthy subjects (10.2±3.6° versus 12.9±2.3°, P=0.003) and patients with DMD (8.6±3.6° versus 10.5±3.6°, P=0.004). AVG results were in between BEL and BH. No significant differences in CL-shear were detected between BEL and BH.
Conclusion
Breath-holding directly affects estimates of peak LV twist, but not CL-shear. Using a free-breathing strategy for the evaluation of cardiac function is important for intrasubject longitudinal studies, intersubject comparisons, and multicenter trials for patients with DMD.
Keywords: free-breathing, tagging, twist, shear angle, torsion, Duchenne muscular dystrophy
INTRODUCTION
Cardiac MRI exams increasingly include the evaluation of quantitative metrics of ventricular performance. Quantitative measures of cardiac function (e.g., strain and twist) obtained from SPAMM (1) or CSPAMM tagging (2,3), HARP (4), or DENSE (5) can be used to stage disease progression and monitor the response to therapy. Conventional cardiac MRI exams, however, require repeated breath-holding, which can be burdensome for some clinical patients. This breath-hold paradigm presents two problems for patients with Duchenne muscular dystrophy (DMD), all of whom develop progressive respiratory impairment and the signs and symptoms of cardiac involvement at an early age (6).
First, breath-holding imparts subtle shifts in the hemodynamic loading of the heart, which consequently impacts quantitative measures of global and regional left ventricular (LV) function (7). In fact, free-breathing may provide a better estimate of normal, ambulatory physiology, whereas breath-holding alters loading conditions and may cause subtle Valsalva or Mueller maneuvering, which alters cardiac function.
Second, while breath-hold studies may be readily obtained when the disease is mild, the studies become more challenging as the disease progresses. Hence, for longitudinal studies it may be judicious to use free-breathing strategies at all time points in anticipation of declining respiratory function. For DMD patients, when breath-held imaging is not feasible, free-breathing techniques, such as navigator gating (8), respiratory bellows gating (9), and multiple signal averages (10) are usually used to minimize respiratory motion artifacts. Current clinical MRI protocols for patients with DMD combine breath-holding and free-breathing strategies, but the quantitative consequences are not well studied.
The purpose of this study was to evaluate the effect of free-breathing on quantitative measure of LV rotational mechanics in healthy subjects and in patients with DMD. The objective was to reevaluate current clinical DMD protocols and to define the best imaging strategies for longitudinal studies.
METHODS
MRI Protocol
The local institutional review board approved this study and all adult subjects provided signed statements of informed consent. Sixteen healthy human subjects (N=16) with no history of cardiovascular or respiratory disease (one female, 27.8±3.9 years) were scanned. In a subsequent study, consent was obtained from parents or legal guardians for pediatric patients with DMD. Five (N=5) patients with DMD were scanned (all male; 11.3±3.7 years; EF: 62.5±4.1%; LGE (±): 3/2). The demographics for each group are summarized in Table 1.
Table 1.
Demographics of Healthy Subjects and Patients with Duchenne Muscular Dystrophy
| Normal | DMD | |
|---|---|---|
| Age [yr] | 27.8±3.9 | 12±4.3 |
| Weight [kg] | 73.1±12.6 | 35.1±10.6 |
| Gender [male/female] | 15/1 | 5/0 |
| Heart rate [bpm] | 59.3±8.3 | 69.3±8.9 |
All subjects were positioned headfirst and supine within the scanner and imaged using a six-element anterior coil array and a six-element posterior coil array with electrocardiogram gating on a 3.0 Tesla (T) scanner (Tim Trio, Siemens Healthcare, Erlangen, Germany). Localizer sequences were used to identify the apical and basal slices in the cardiac short-axis (SA) plane. Global LV function was measured using balanced steady-state free precession (bSSFP) cine images collected with a 6mm slice thickness and a 4- mm gap between slices, in parallel LV SA planes.
A cardiac gated spoiled gradient echo sequence was modified to support 1-1 binomially weighted ORI-SPAMM (11), which was developed to eliminate the effects of off-resonance accrued during motion encoding. ORI-SPAMM modifies the original SPAMM tagging prep to include a 180° refocusing pulse and a split motion encoding gradient. This 180° pulse refocuses off-resonance accrued during the tagging preparation, which can lead to errors in quantitative measures of LV strain and rotation. ORI-SPAMM tagged images of the LV short-axis were acquired at the most basal and apical slice locations for which the myocardium retained an annular shape during the entire cardiac cycle. The following parameters were used: 280–330 × 280–330mm field-of-view, 6mm slice thickness, 192 × 192 acquisition matrix, 395Hz/pixel receiver bandwidth, echo time/repetition time (TE/TR)=2.33–2.39/4.71–4.83 ms, eight phase encode lines per segment, 12° imaging flip angle, 8mm tag spacing, two-fold GRAPPA (12) parallel imaging acceleration (24 reference lines).
Cine ORI-SPAMM tagged images were acquired using both horizontal and vertical tagging in separate acquisitions for each subject during breath-holding (BH), free-breathing with averaging (AVG), and free-breathing with respiratory bellows gating (BEL). The BH scans were acquired during an end expiratory breath-hold of 15 heartbeats (12.5±2.1 s depending upon heart rate). The free-breathing images were acquired during free-breathing with four signal averages. The BEL scans were acquired prospectively during free-breathing with the respiratory bellows gating set to acquire during the window of 30–40% expiration. Total scan time for the BEL sequence was 45 to 65 s. The scan order for horizontal and vertical tagging of the apex and base was randomized for each set of scans for each subject, to minimize the order effects.
Fourier Analysis of STimulated echoes (FAST)
FAST analysis is a semiautomated image processing method designed to quantify global LV rotation from tagged MR images. The main principle of the FAST method is that rotation in image space has a one to one correspondence with rotation in Fourier space, but in Fourier space the tagging information is focused into stimulated echoes (i.e., harmonic peaks), which are simpler to track than multiple tag lines. FAST has been validated and shown to compare favorably with conventional estimates of LV twist from cardiac tagged images, but with significantly reduced user interaction time during postprocessing (13) and high intrascan reproducibility (14).
FAST was applied to images collected at both the apex and base of the heart to measure the apical (Φapex) and basal (Φbase) rotation in degrees. The difference between Φapex and Φbase is defined as twist (Eq. [1]) (14).
| [1] |
The LV circumferential-longitudinal shear-angle (CL-shear angle) was also measured and is defined as (15):
| [2] |
where ρ is the epicardial radius of the apex or base (mm), and D is the distance between the apical and basal slices (mm). Estimates of apical and basal epicardial radii were made from averaging the end systolic semimajor and semiminor axes of the ellipses used to mask the LV during FAST processing. Peak CL-shear angle was calculated as an alternate measure of LV rotational mechanics that normalizes for heart size and selection of imaging planes (16).
Statistical Analysis
The Wilcoxon signed rank test was used to test for the difference in peak LV twist and peak LV CL-shear angle between the two groups. Bland-Altman analysis was used to compute the bias and 95% confidence intervals for peak LV twist and peak LV CL-shear angle for comparisons of each respiratory motion compensation technique. The Bland-Altman bias (median of the difference between groups) and 95% confidence intervals were calculated using bootstrap sampling 1000 times with replacement due to the limited sample size and non-Gaussian distribution.
RESULTS
Image Quality
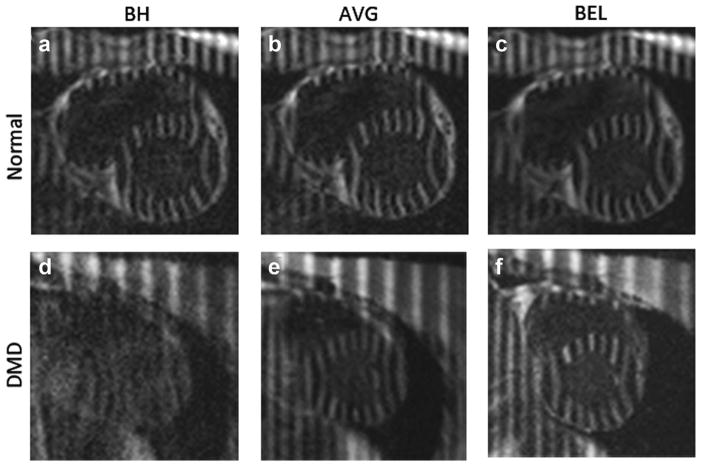
Figure 1 demonstrates the ORI-SPAMM tagged image quality in the LV short axis view for healthy subjects (Figs. 1A–C) and DMD patients (Figs. 1D–F). For the healthy subjects, the image quality is good and the BH (A) and BEL (C) images are comparable. Figure 1D is a typical case wherein the patient with DMD cannot breath-hold well, resulting in blurred images, whereas AVG (Fig. 1E) partially mitigates motion blurring and BEL (Fig. 1F) demonstrates substantially improved image quality.
FIG. 1.
Tagged LV images in the short axis view for a healthy volunteer (A–C) and a patient with DMD (D–F) acquired with BH (A,D), AVG (B,E) and BEL (C,F) respiratory motion compensation.
LV Rotational Mechanics
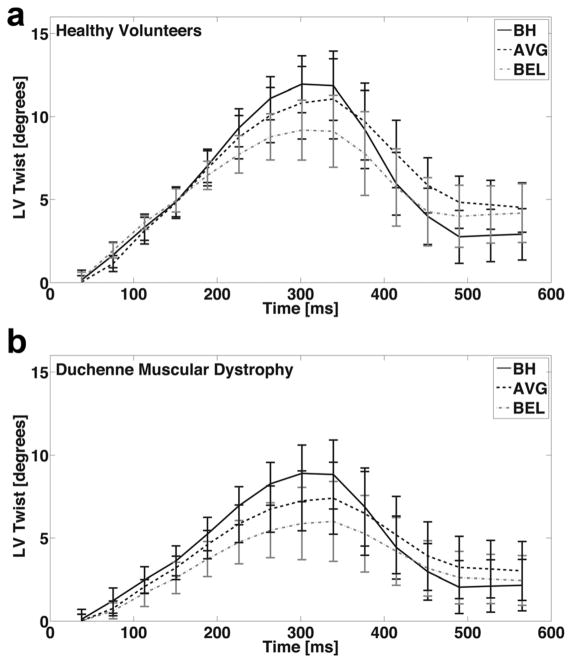
Figure 2A demonstrates the mean LV twist curve from 16 healthy subjects for BH, AVG, and BEL data. Errors bars represent 1 SD and characterize within group physiologic variance. For healthy subjects, the mean peak LV twist measurements were 12.9±2.3° (BH), 11.3±3.8° (AVG), and 10.0±3.6° (BEL). BEL estimates of peak LV twist were 22.5% lower and significantly different than BH estimates (P=0.003).
FIG. 2.
Mean LV twist for healthy subjects (A) and patients with DMD (B) for breath-held (BH, solid), free-breathing with averaging (AVG, dashed) and bellows gated (BEL, dash-dotted) respiratory motion compensation. Error bars are standard error of the mean for clarity and characterize within group physiologic variance. BH LV twist is generally higher compared with AVG or BEL, especially at its peak.
Figure 2B demonstrates the mean LV twist curve from five DMD patients. For patients with DMD, the mean peak LV twist measurements were 10.5±3.6° (BH), 9.3±3.4° (AVG), and 8.5±3.6° (BEL); and BEL was 19.0% lower and significantly different than BH (P=0.004). Hence, a similar trend was observed in both healthy subjects and patients with DMD wherein BH had the highest peak LV twist values, AVG had intermediate peak LV twist values, and BEL had the lowest peak LV twist values. The LV rotational and geometric measures used to estimate LV twist and CL-shear are summarized in Table 2.
Table 2.
Measurements of LV Rotational Mechanics in Healthy Subjects and in Patients with Duchenne Muscular Dystrophy
| Normal
|
DMD
|
|||||
|---|---|---|---|---|---|---|
| BH | AVG | BEL | BH | AVG | BEL | |
| Mean peak LV twist [deg] | 12.9±2.3 | 11.3±3.8 | 10.2±3.6a | 10.5±3.6 | 9.3±3.4 | 8.6±3.6a |
| Mean peak CL-shear angle [deg] | 6.4±1.7 | 6.2±1.4 | 5.9±1.6 | 5.9±1.7 | 5.7±1.4 | 5.6±2.1 |
| Mean peak Apical rotation [deg] | 8.9±2.9 | 8.1±3.0 | 7.2±3.8a | 7.1±3.1 | 6.4±3.4 | 5.8±3.7 |
| Mean peak Basal rotation [deg] | −4.4±1.8 | −3.2±2.4 | −3.0±1.6 | −3.4±1.7 | −2.9±2.3 | −2.8±1.7 |
| LV apical Epicardial radius [mm] | 23.3±2.5 | 25.0±3.1 | 25.9±3.4a | 17.1±3.1 | 18.4±3.5 | 20.2±4.6a |
| LV basal Epicardial radius [mm] | 32.3±2.3 | 32.9±2.4 | 33.1±2.5a | 27.0±3.0 | 28.7±3.3 | 30.4±4.1a |
| Distance between Apex and Base [cm] | 5.0±1.9 | 4.0±1.2 | ||||
Indicates statistical difference compared to BH (P<0.05)
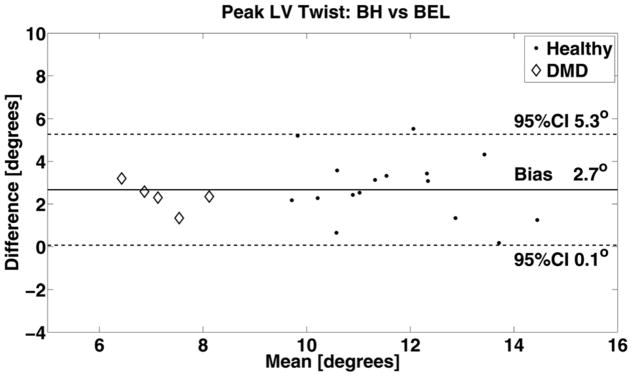
Bland-Altman analysis of peak LV twist between each respiratory compensation method are summarized: BH versus AVG analysis resulted in a bias of 1.5° (0.1°, 2.9°) and AVG versus BEL resulted in a bias of 1.2° and (0.1°, 2.3°) indicating moderate agreement between the two respiratory compensation method. Notably, BH peak LV twist was greater than BEL peak LV twist for all subjects. A similar trend was seen for AVG versus BEL. BH versus BEL results for the pool of healthy subjects (dots) and DMD patients (diamonds) are demonstrated in Figure 3, which shows a median bias of 2.7° and 95% CI (0.1°, 5.3°) indicating a non-negligible increase of BH peak LV twist compared with BEL.
FIG. 3.
Bland-Altman analysis of peak LV twist derived from breath-held (BH) versus free-breathing with bellow gating (BEL) indicates a non-negligible difference and a bias of 2.7° and a 95% confidence interval of (0.1°, 5.3°). The difference likely arises from respiratory induced changes in loading conditions between BH and BEL.
LV Geometry
The mean apical and basal epicardial radii measured in an end-systolic cardiac frame were reported in Table 2. The only significant differences observed in the radius data were between the decreased apical epicardial radii for BH compared with BEL (P=0.007) and the similarly decreased basal epicardial radius for BH compared BEL (P=0.006); all other differences were not significant with P>0.05 despite an apparent trend for an increase in radii from BH to AVG to BEL.
CL-Shear Angle
The peak LV CL-shear angle measurements were 6.4±1.7° (BH), 6.2±1.4° (AVG), and 5.9±1.6° (BEL) for healthy subjects and were not significantly different between groups. Similarly, for patients with DMD the peak LV CL-shear angle measurements were 5.9±1.7° (BH), 5.7±1.4° (AVG), and 5.6±2.1° (BEL) and were not significantly different. Bland-Altman analysis of the pooled peak CL-shear data for BH versus AVG resulted in a bias of 0.1° (−0.5°, 1.6°), AVG versus BEL resulted in bias of 0.6° (0.1°, 1.6°), and BH versus BEL resulted in a bias of 0.8° (−0.4°, 2.1°). These results indicate good agreement for estimates of peak LV CL-shear between the different respiratory motion compensation methods.
DISCUSSION
Breath-holding alters cardiovascular loading conditions compared with free-breathing, likely as a consequence of subtle changes in intrathoracic pressure. Cardiac MRI performed during free-breathing conditions may produce measurements that are less prone to measurement bias as a consequence of inadvertent, irreproducible, and subtle Valsalva (17) or Mueller (18) maneuvers. Notably, the differences in peak LV twist between BH and BEL are larger than those observed in a previous intraexam reproducibility study (14), which showed differences of −0.6° compared with 2.3° found in this study. This indicates that breath-hold conditions are an important consideration in longitudinal studies. In patients with DMD, due to progressive muscle weakness, breath-holding becomes increasingly difficult. Because the quality of breath-holding cannot be controlled at each time point in a longitudinal study of patients with DMD, free-breathing approaches are a judicious consideration. Alternately, because measures of peak LV CL-shear appear to be less effected by the breath-hold conditions, this measure may be a more consistent measure of LV rotational mechanics in these patients.
This study demonstrated that BEL estimates of peak LV twist were significantly smaller than BH estimates, however, AVG peak LV twist estimates were only slightly lower than BH peak LV twist values. This trend in mean peak LV twist values is evident in Table 2. Similarly, quantitative measures like radial, circumferential, and longitudinal strain derived from navigator-gated 3D cine DENSE have been shown to correlate well with, but are lower than breath-held strain values (19). Likewise, HARP strain estimates during free-breathing are decreased compared with breath-holding values (20). These differences all likely arise from respiratory induced differences in loading conditions.
The results also show a decrease in CL-shear angle from BH to AVG to BEL in both healthy subjects and DMD patients, but the percent change is lower than for LV twist. This arises from a significant increase in the apical and basal epicardial radii in conjunction with the significant decrease in apical rotation. The observed increase in end systolic epicardial radius during BEL is consistent with an increase in afterload, which has previously been shown to accord with a decrease in torsion (21). The concomitant change in loading conditions and LV volumes (i.e., radii) underlies the differences observed between BH and BEL peak LV twist measurements and contributes to the fact that there was no significant difference in the peak CL-shear angle data between the different respiratory motion compensation methods. This indicates that CL-shear angle appears to pseudonormalize and may be less sensitive than LV twist to different respiratory compensation methods. Note, however, that CL-shear may be a better measure of LV rotational mechanics for longitudinal studies when breath-hold conditions cannot be controlled longitudinally.
Our results suggest that LV twist and CL-shear are reduced in boys with DMD relative to non–age-matched healthy subjects. Several previous studies of patients with DMD have used strain as a measure of regional ventricular function (22,23). Ashford et al. (24) reported decreases in global LV torsion (twist divided by base-to-apex slice distance) and decreases in global circumferential strain for boys with DMD, but only the strain finding was statistically significant relative to age-matched controls. The strain finding was also corroborated by the work of Hor et al. (22). The impact of free-breathing compared with breath-holding on DMD strain analysis, however, is not fully understood and requires future evaluation.
This study occurred in two stages wherein healthy subjects were evaluated first and we subsequently evaluated a cohort of patients with DMD. Hence, direct comparisons between the two groups were not made. Nevertheless, very similar trends are seen in both groups for peak LV twist as a function of breath-holding condition. Peak LV twist values were observed to be lower in patients with DMD compared with healthy subjects, but there is also a notable age difference between the two groups. Preliminary work (25) indicates that LV twist increases with age, whereas CL-shear is relatively constant over five decades. Ideally, a group of age-matched pediatric controls would be enrolled for a comparative study, but this has proven to be challenging.
Although not widely available at all imaging centers, nor for all MRI pulse sequences, respiratory motion compensation with navigator echoes is an attractive option, but was not available for this study. Moving forward consideration will be given to image-based navigators (26), which can avoid the set-up time associated with both bellows and navigator echoes.
In conclusion, breath-holding significantly affects estimates of peak LV twist, but not CL-shear. A significant decrease in LV twist (22.5% in healthy subjects and 19.0% patients with DMD) between BH and BEL was detected, making it a non-negligible factor during diagnosis and longitudinal follow-up. When using quantitative imaging biomarkers of LV rotational mechanics to monitoring disease progression or the response to therapy, especially in patients with DMD for whom decline in respiratory function is certain, it will be important to use a free-breathing strategy for all studies to facilitate intrasubject longitudinal studies, intersubject comparisons, and multicenter trials. Current DMD protocols that combine breath-holding and free-breathing strategies could introduce measurement bias and should be reevaluated.
Limitations
The effects of through-plane motion should also be considered as a possible confounder. Stuber et al. previously presented a slice-following method to account for through plane motion (27), which could be considered in a future implementation. The work of Brotman et al. highlights that a reduction of peak LV torsion by ~1° is seen when through-plane motion is accounted for using slice-following (28).
Acknowledgments
Grant sponsor: AHA; Grant numbers: 11-PRE-6080005, 13-BGIA- 14530010; Grant sponsor: Center for Duchenne Muscular Dystrophy at UCLA.
M.L.R. was supported by the AHA (11-PRE-6080005), and D.B.E. was funded by UCLA’s Center for Duchenne Muscular Dystrophy and the AHA (13-BGIA-14530010).
References
- 1.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171:841–845. doi: 10.1148/radiology.171.3.2717762. [DOI] [PubMed] [Google Scholar]
- 2.Ennis DB, Epstein FH, Kellman P, Fananapazir L, McVeigh ER, Arai AE. Assessment of regional systolic and diastolic dysfunction in familial hypertrophic cardiomyopathy using MR tagging. Magn Reson Med. 2003;50:638–642. doi: 10.1002/mrm.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer SE, McKinnon GC, Maier SE, Boesiger P. Improved myocardial tagging contrast. Magn Reson Med. 1993;30:191–200. doi: 10.1002/mrm.1910300207. [DOI] [PubMed] [Google Scholar]
- 4.Osman NF, Kerwin WS, McVeigh ER, Prince JL. Cardiac motion tracking using CINE harmonic phase (HARP) magnetic resonance imaging. Magn Reson Med. 1999;42:1048–1060. doi: 10.1002/(sici)1522-2594(199912)42:6<1048::aid-mrm9>3.0.co;2-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aletras AH, Ding S, Balaban RS, Wen H. DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson. 1999;137:247–252. doi: 10.1006/jmre.1998.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonds AK. Respiratory complications of the muscular dystrophies. Semin Respir Crit Care Med. 2002;23:231–238. doi: 10.1055/s-2002-33031. [DOI] [PubMed] [Google Scholar]
- 7.Herzka DA, Derbyshire JA, Kellman P, McVeigh ER. Single heartbeat cardiac tagging for the evaluation of transient phenomena. Magn Reson Med. 2005;54:1455–1464. doi: 10.1002/mrm.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X, Kim SG. Reduction of signal fluctuation in functional MRI using navigator echoes. Magn Reson Med. 1994;31:495–503. doi: 10.1002/mrm.1910310505. [DOI] [PubMed] [Google Scholar]
- 9.Santelli C, Nezafat R, Goddu B, Manning WJ, Smink J, Kozerke S, Peters DC. Respiratory bellows revisited for motion compensation: preliminary experience for cardiovascular MR. Magn Reson Med. 2011;65:1097–1102. doi: 10.1002/mrm.22687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung T. Assessment of cardiovascular anatomy in patients with congenital heart disease by magnetic resonance imaging. Pediatr Cardiol. 2000;21:18–26. doi: 10.1007/s002469910004. [DOI] [PubMed] [Google Scholar]
- 11.Reyhan M, Natsuaki Y, Ennis DB. Off-resonance insensitive complementary SPAtial Modulation of Magnetization (ORI-CSPAMM) for quantification of left ventricular twist. J Magn Reson Imaging. 2014;39:339–345. doi: 10.1002/jmri.24154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 13.Reyhan M, Natsuaki Y, Ennis DB. Fourier analysis of STimulated echoes (FAST) for the quantitative analysis of left ventricular twist. J Magn Reson Imaging. 2012;35:587–593. doi: 10.1002/jmri.22863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyhan M, Kim HJ, Brown MS, Ennis DB. Intra- and interscan reproducibility using Fourier Analysis of STimulated Echoes (FAST) for the rapid and robust quantification of left ventricular twist. J Magn Reson Imaging. 2014;39:463–468. doi: 10.1002/jmri.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russel IK, Tecelao SR, Kuijer JP, Heethaar RM, Marcus JT. Comparison of 2D and 3D calculation of left ventricular torsion as circumferential-longitudinal shear angle using cardiovascular magnetic resonance tagging. J Cardiovasc Magn Reson. 2009;11:8. doi: 10.1186/1532-429X-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young AA, Cowan BR. Evaluation of left ventricular torsion by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:49. doi: 10.1186/1532-429X-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorlin R, Knowles JH, Storey CF. The Valsalva maneuver as a test of cardiac function; pathologic physiology and clinical significance. Am J Med. 1957;22:197–212. doi: 10.1016/0002-9343(57)90004-9. [DOI] [PubMed] [Google Scholar]
- 18.Condos WR, Jr, Latham RD, Hoadley SD, Pasipoularides A. Hemodynamics of the Mueller maneuver in man: right and left heart micromanometry and Doppler echocardiography. Circulation. 1987;76:1020–1028. doi: 10.1161/01.cir.76.5.1020. [DOI] [PubMed] [Google Scholar]
- 19.Zhong X, Spottiswoode BS, Meyer CH, Kramer CM, Epstein FH. Imaging three-dimensional myocardial mechanics using navigator-gated volumetric spiral cine DENSE MRI. Magn Reson Med. 2010;64:1089–1097. doi: 10.1002/mrm.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampath S, Derbyshire JA, Atalar E, Osman NF, Prince JL. Real-time imaging of two-dimensional cardiac strain using a harmonic phase magnetic resonance imaging (HARP-MRI) pulse sequence. Magn Reson Med. 2003;50:154–163. doi: 10.1002/mrm.10509. [DOI] [PubMed] [Google Scholar]
- 21.Dong SJ, Hees PS, Huang WM, Buffer SA, Jr, Weiss JL, Shapiro EP. Independent effects of preload, afterload, and contractility on left ventricular torsion. Am J Physiol. 1999;277(Pt 2):H1053–H1060. doi: 10.1152/ajpheart.1999.277.3.H1053. [DOI] [PubMed] [Google Scholar]
- 22.Hor KN, Wansapura J, Markham LW, Mazur W, Cripe LH, Fleck R, Benson DW, Gottliebson WM. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. J Am Coll Cardiol. 2009;53:1204–1210. doi: 10.1016/j.jacc.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazur W, Hor KN, Germann JT, et al. Patterns of left ventricular remodeling in patients with Duchenne Muscular Dystrophy: a cardiac MRI study of ventricular geometry, global function, and strain. Int J Cardiovasc Imaging. 2012;28:99–107. doi: 10.1007/s10554-010-9781-2. [DOI] [PubMed] [Google Scholar]
- 24.Ashford MW, Jr, Liu W, Lin SJ, Abraszewski P, Caruthers SD, Connolly AM, Yu X, Wickline SA. Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation. 2005;112:2462–2467. doi: 10.1161/CIRCULATIONAHA.104.516716. [DOI] [PubMed] [Google Scholar]
- 25.Reyhan ML, Li M, Gupta H, Lloyd SG, Dell’Italia LJ, Kim HJ, Denney T, Ennis DB. Left ventricular twist, but not circumferential-longitudinal shear angle, increases with increasing age in normal subjects. SCMR Conf Proc. 2013;15(Suppl 1) [Google Scholar]
- 26.Kellman P, Chefd’hotel C, Lorenz CH, Mancini C, Arai AE, McVeigh ER. Fully automatic, retrospective enhancement of real-time acquired cardiac cine MR images using image-based navigators and respiratory motion-corrected averaging. Magn Reson Med. 2008;59:771–778. doi: 10.1002/mrm.21509. [DOI] [PubMed] [Google Scholar]
- 27.Stuber M, Spiegel MA, Fischer SE, Scheidegger MB, Danias PG, Pedersen EM, Boesiger P. Single breath-hold slice-following CSPAMM myocardial tagging. Magma. 1999;9:85–91. doi: 10.1007/BF02634597. [DOI] [PubMed] [Google Scholar]
- 28.Brotman D, Zhang Z, Sampath S. Effect of through-plane motion on left ventricular rotation: a study using slice-following harmonic phase imaging. Magn Reson Med. 2013;69:1421–1429. doi: 10.1002/mrm.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]