Figure 3. Selective sensitivity of cNCCs to p53 activation (act.) and DDX21 levels.
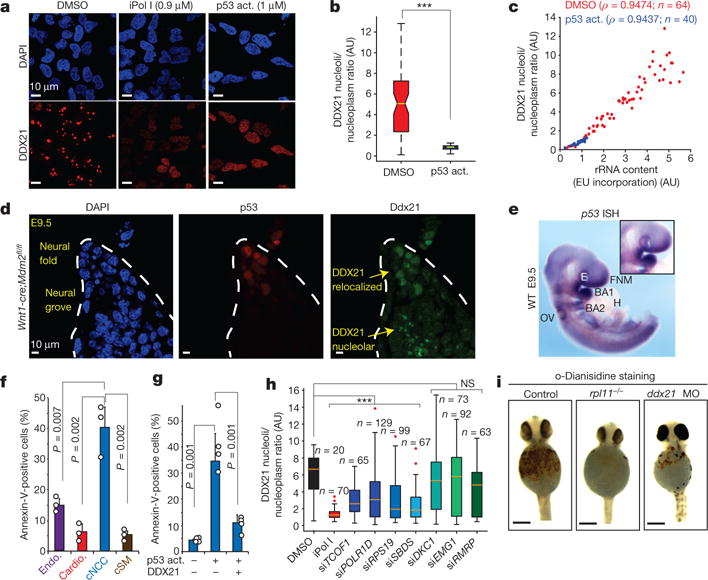
a, Representative immunofluorescence images of DAPI and DDX21 stainings in human cNCCs treated with DMSO, iPol I or the p53 activator (NSC146109). n = 3 biologically independent experiments. b, c, Quantification of DDX21 nucleolar/nucleoplasmic ratio (b) and the relationship between rRNA synthesis and DDX21 localization (c) in cNCCs after 12 h treatment with DMSO or NSC146109. Cells were collected from three biologically independent experiments. Boxes represent median value and 25th and 75th percentiles, whiskers are minimum to maximum. ***P < 0.001, two-sided Wilcoxon–Mann–Whitney test. d, Immunofluorescence staining of p53 and DDX21 in sections from the dorsal neural tube of Wnt1-cre;Mdm2fl/fl embryonic day (E)9.5 mouse embryos. Dotted lines represent the neural fold. n = 5 independent animals per genotype. e, Representative picture of whole-mount in situ hybridization (ISH) for p53 mRNA in E9.5 embryos. Inset highlights the first brachial arch (BA1). WT, wild type; E, eye; FNM, frontonasal mass; H, heart; OV, otic vesicle. n = 4 independent animals. f, Differentiation of human ES cells into the indicated cell types (Endo., endothelial cells; Cardio., cardiomyocytes; cSM, cNCC-derived smooth muscle). Sensitivity to p53-mediated apoptosis after treatment with NSC146109 for 12–16 h was quantified by fluorescence-activated cell sorting (FACS). Bars are from n = 3 biologically independent experiments; error bars, s.e.m. g, Human cNCC wild type or overexpressing GFP–DDX21 were treated with NSC146109, followed by FACS analyses of annexin V staining. Bars are from n = 4 biologically independent experiments; error bars, s.e.m. For f and g P values, two-sided, unpaired t-test. h, Box plot represents quantification of DDX21 nucleolar/nucleoplasmic ratio for indicated siRNAs. Boxes represent median value and 25th and 75th percentiles, whiskers are minimum to maximum, crosses are outliers. ***P < 0.001, two-sided Wilcoxon–Mann–Whitney test. NS, not significant. Cells were collected from three biologically independent experiments. i, Haemoglobin staining with o-dianisidine of control, rpl11−/−, and ddx21 morpholino-injected zebrafish embryos at 24 h postfertilization. Embryos were collected from n = 3 independent matings.
