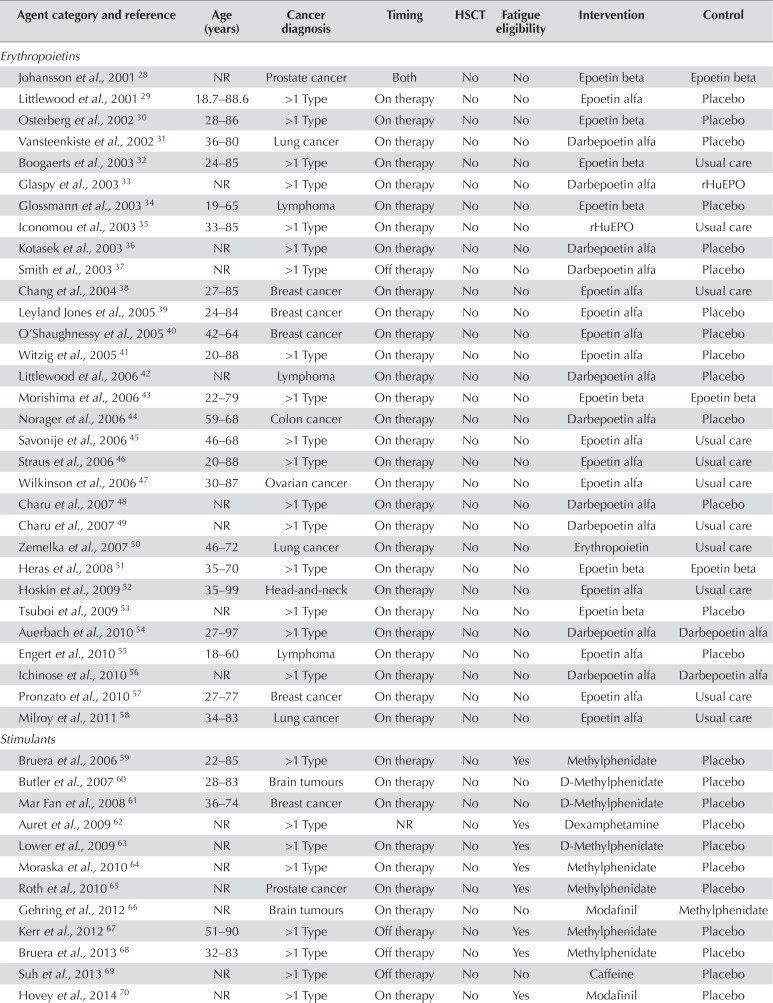
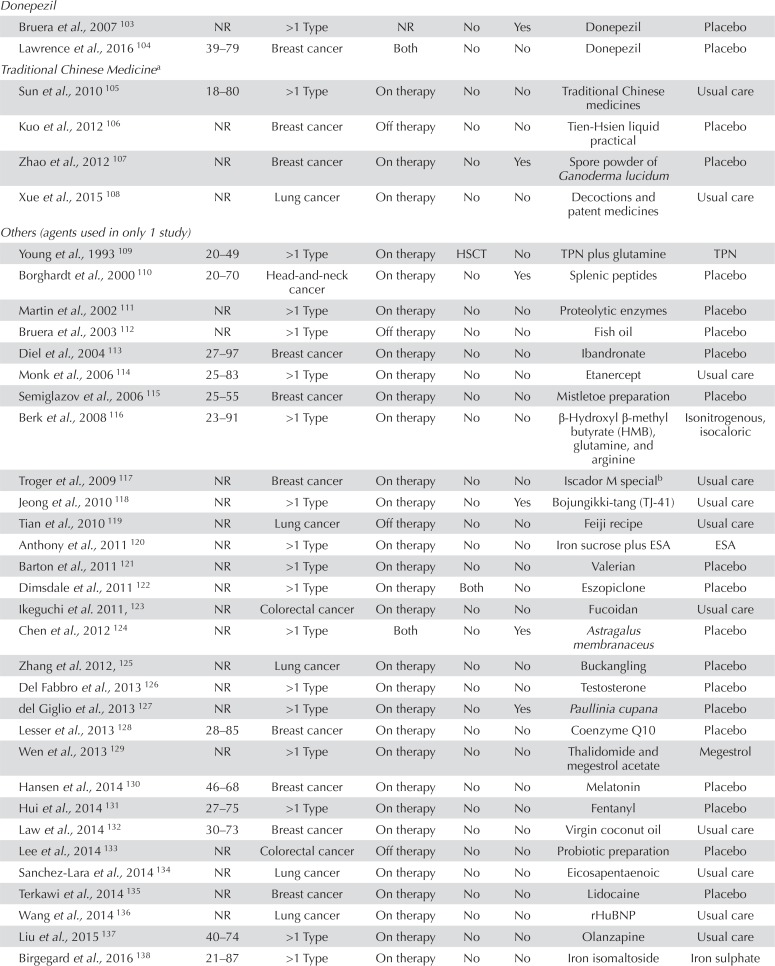
TABLE IV.
Details of the 117 included studies
| Agent category and reference | Age (years) | Cancer diagnosis | Timing | HSCT | Fatigue eligibility | Intervention | Control |
|---|---|---|---|---|---|---|---|
| Erythropoietins | |||||||
| Johansson et al., 2001 28 | NR | Prostate cancer | Both | No | No | Epoetin beta | Epoetin beta |
| Littlewood et al., 2001 29 | 18.7–88.6 | >1 Type | On therapy | No | No | Epoetin alfa | Placebo |
| Osterberg et al., 2002 30 | 28–86 | >1 Type | On therapy | No | No | Epoetin beta | Placebo |
| Vansteenkiste et al., 2002 31 | 36–80 | Lung cancer | On therapy | No | No | Darbepoetin alfa | Placebo |
| Boogaerts et al., 2003 32 | 24–85 | >1 Type | On therapy | No | No | Epoetin beta | Usual care |
| Glaspy et al., 2003 33 | NR | >1 Type | On therapy | No | No | Darbepoetin alfa | rHuEPO |
| Glossmann et al., 2003 34 | 19–65 | Lymphoma | On therapy | No | No | Epoetin beta | Placebo |
| Iconomou et al., 2003 35 | 33–85 | >1 Type | On therapy | No | No | rHuEPO | Usual care |
| Kotasek et al., 2003 36 | NR | >1 Type | On therapy | No | No | Darbepoetin alfa | Placebo |
| Smith et al., 2003 37 | NR | >1 Type | Off therapy | No | No | Darbepoetin alfa | Placebo |
| Chang et al., 2004 38 | 27–85 | Breast cancer | On therapy | No | No | Epoetin alfa | Usual care |
| Leyland Jones et al., 2005 39 | 24–84 | Breast cancer | On therapy | No | No | Epoetin alfa | Placebo |
| O’Shaughnessy et al., 2005 40 | 42–64 | Breast cancer | On therapy | No | No | Epoetin alfa | Placebo |
| Witzig et al., 2005 41 | 20–88 | >1 Type | On therapy | No | No | Epoetin alfa | Placebo |
| Littlewood et al., 2006 42 | NR | Lymphoma | On therapy | No | No | Darbepoetin alfa | Placebo |
| Morishima et al., 2006 43 | 22–79 | >1 Type | On therapy | No | No | Epoetin beta | Epoetin beta |
| Norager et al., 2006 44 | 59–68 | Colon cancer | On therapy | No | No | Darbepoetin alfa | Placebo |
| Savonije et al., 2006 45 | 46–68 | >1 Type | On therapy | No | No | Epoetin alfa | Usual care |
| Straus et al., 2006 46 | 20–88 | >1 Type | On therapy | No | No | Epoetin alfa | Usual care |
| Wilkinson et al., 2006 47 | 30–87 | Ovarian cancer | On therapy | No | No | Epoetin alfa | Usual care |
| Charu et al., 2007 48 | NR | >1 Type | On therapy | No | No | Darbepoetin alfa | Placebo |
| Charu et al., 2007 49 | NR | >1 Type | On therapy | No | No | Darbepoetin alfa | Usual care |
| Zemelka et al., 2007 50 | 46–72 | Lung cancer | On therapy | No | No | Erythropoietin | Usual care |
| Heras et al., 2008 51 | 35–70 | >1 Type | On therapy | No | No | Epoetin beta | Epoetin beta |
| Hoskin et al., 2009 52 | 35–99 | Head-and-neck | On therapy | No | No | Epoetin alfa | Usual care |
| Tsuboi et al., 2009 53 | NR | >1 Type | On therapy | No | No | Epoetin beta | Placebo |
| Auerbach et al., 2010 54 | 27–97 | >1 Type | On therapy | No | No | Darbepoetin alfa | Darbepoetin alfa |
| Engert et al., 2010 55 | 18–60 | Lymphoma | On therapy | No | No | Epoetin alfa | Placebo |
| Ichinose et al., 2010 56 | NR | >1 Type | On therapy | No | No | Darbepoetin alfa | Darbepoetin alfa |
| Pronzato et al., 2010 57 | 27–77 | Breast cancer | On therapy | No | No | Epoetin alfa | Usual care |
| Milroy et al., 2011 58 | 34–83 | Lung cancer | On therapy | No | No | Epoetin alfa | Usual care |
| Stimulants | |||||||
| Bruera et al., 2006 59 | 22–85 | >1 Type | On therapy | No | Yes | Methylphenidate | Placebo |
| Butler et al., 2007 60 | 28–83 | Brain tumours | On therapy | No | No | D-Methylphenidate | Placebo |
| Mar Fan et al., 2008 61 | 36–74 | Breast cancer | On therapy | No | No | D-Methylphenidate | Placebo |
| Auret et al., 2009 62 | NR | >1 Type | NR | No | Yes | Dexamphetamine | Placebo |
| Lower et al., 2009 63 | NR | >1 Type | On therapy | No | Yes | D-Methylphenidate | Placebo |
| Moraska et al., 2010 64 | NR | >1 Type | On therapy | No | Yes | Methylphenidate | Placebo |
| Roth et al., 2010 65 | NR | Prostate cancer | On therapy | No | Yes | Methylphenidate | Placebo |
| Gehring et al., 2012 66 | NR | Brain tumours | On therapy | No | No | Modafinil | Methylphenidate |
| Kerr et al., 2012 67 | 51–90 | >1 Type | Off therapy | No | Yes | Methylphenidate | Placebo |
| Bruera et al., 2013 68 | 32–83 | >1 Type | Off therapy | No | Yes | Methylphenidate | Placebo |
| Suh et al., 2013 69 | NR | >1 Type | Off therapy | No | No | Caffeine | Placebo |
| Hovey et al., 2014 70 | NR | >1 Type | On therapy | No | Yes | Modafinil | Placebo |
| Spathis et al., 2014 71 | NR | Lung cancer | On therapy | No | Yes | Modafinil | Placebo |
| Berenson et al., 2015 72 | 43–85 | Multiple myeloma | On therapy | No | Yes | Armodafinil | Placebo |
| Page et al., 2015 73 | 20–79 | Brain tumours | On therapy | No | No | Armodafinil | Placebo |
| Richard et al., 2015 74 | NR | Prostate cancer | On therapy | No | Yes | Methylphenidate | Placebo |
| Heckler et al., 2016 75 | NR | >1 Type | Off therapy | No | No | Armodafinil | Placebo |
| Jean-Pierre et al., 2016 76 | 18–90 | >1 Type | Both | No | Yes | Modafinil | Placebo |
| Lee et al., 2016 77 | 19–79 | Brain tumours | On therapy | No | No | Armodafinil | Placebo |
| Corticosteroids | |||||||
| Inoue et al., 2003 78 | 28–78 | >1 Type | On therapy | No | No | Dexamethasone | Placebo |
| Zarger-Shoshtari et al. 2009, 79 | 34–92 | Colorectal cancer | On therapy | No | No | Dexamethasone | Placebo |
| Yennurajalingam et al., 2013 80 | 29–89 | >1 Type | Both | No | Yes | Dexamethasone | Placebo |
| Paulsen et al., 2014 81 | NR | >1 Type | Both | No | Yes | Methylprednisolone | Placebo |
| Eguchi et al., 2015 82 | 46–84 | >1 Type | Off therapy | No | No | Methylprednisolone | Placebo |
| L-Carnitine | |||||||
| Cruciani et al., 2009 83 | 53.7–84.6 | >1 Type | Both | No | Yes | L-Carnitine | Placebo |
| Mantovani et al., 2010 84 | NR | >1 Type | Both | No | No | L-Carnitine | Nutritional supplement |
| Cruciani et al., 2012 85 | NR | >1 Type | Both | No | Yes | L-Carnitine | Placebo |
| Kraft et al., 2012 86 | NR | Pancreatic cancer | Both | No | No | L-Carnitine | Placebo |
| Hershman et al., 2013 87 | 26–80 | Breast cancer | On therapy | No | No | Acetyl-L-carnitine | Placebo |
| Iwase et al., 2016 88 | 22–70 | Breast cancer | Both | No | Yes | L-Carnitine | Usual care |
| Antidepressants | |||||||
| Capuron et al., 2002 89 | 25–74 | Malignant melanoma | On therapy | No | No | Paroxetine | Placebo |
| Morrow et al., 2003 90 | 23–87 | >1 Type | On therapy | No | Yes | Paroxetine | Placebo |
| Roscoe et al., 2005 91 | 31–79 | Breast cancer | On therapy | No | Yes | Paroxetine | Placebo |
| Stockler et al., 2007 92 | NR | >1 Type | On therapy | No | No | Sertraline | Placebo |
| Heras et al., 2013 93 | 32–89 | >1 Type | On therapy | No | Yes | Paroxetine | Placebo |
| Appetite stimulant | |||||||
| Simons et al., 1996 94 | NR | >1 Type | Off therapy | No | No | Medroxyprogesterone acetate | Placebo |
| De Conno et al., 1998 95 | NR | >1 Type | Off therapy | No | No | Megestrol | Placebo |
| Westman et al., 1999 96 | 37–89 | >1 Type | On therapy | No | No | Megestrol acetate | Placebo |
| American ginseng | |||||||
| Barton et al., 2010 97 | NR | >1 Type | On therapy | No | Yes | American ginseng | Placebo |
| Barton et al., 2013 98 | NR | >1 Type | Both | No | Yes | American ginseng | Placebo |
| Adenosine 5′-triphosphate (ATP) | |||||||
| Agteresch et al., 2000 99 | NR | Lung cancer | Off therapy | No | No | ATP | Usual care |
| Beijer et al., 2010 100 | NR | >1 Type | Both | No | No | ATP | Usual care |
| Celecoxib | |||||||
| Cerchietti et al., 2007 101 | 44–90 | Lung cancer | Off therapy | No | No | Celecoxib | Placebo and fish oil |
| Maccio et al., 2012 102 | NR | >1 Type | Both | No | No | Celecoxib, megestrol acetate, L-carnitine, and antioxidants | Megestrol acetate |
| Donepezil | |||||||
| Bruera et al., 2007 103 | NR | >1 Type | NR | No | Yes | Donepezil | Placebo |
| Lawrence et al., 2016 104 | 39–79 | Breast cancer | Both | No | No | Donepezil | Placebo |
| Traditional Chinese Medicinea | |||||||
| Sun et al., 2010 105 | 18–80 | >1 Type | On therapy | No | No | Traditional Chinese medicines | Usual care |
| Kuo et al., 2012 106 | NR | Breast cancer | Off therapy | No | No | Tien-Hsien liquid practical | Placebo |
| Zhao et al., 2012 107 | NR | Breast cancer | On therapy | No | Yes | Spore powder of Ganoderma lucidum | Placebo |
| Xue et al., 2015 108 | NR | Lung cancer | On therapy | No | No | Decoctions and patent medicines | Usual care |
| Others (agents used in only 1 study) | |||||||
| Young et al., 1993 109 | 20–49 | >1 Type | On therapy | HSCT | No | TPN plus glutamine | TPN |
| Borghardt et al., 2000 110 | 20–70 | Head-and-neck cancer | On therapy | No | Yes | Splenic peptides | Placebo |
| Martin et al., 2002 111 | NR | >1 Type | On therapy | No | No | Proteolytic enzymes | Placebo |
| Bruera et al., 2003 112 | NR | >1 Type | Off therapy | No | No | Fish oil | Placebo |
| Diel et al., 2004 113 | 27–97 | Breast cancer | On therapy | No | No | Ibandronate | Placebo |
| Monk et al., 2006 114 | 25–83 | >1 Type | On therapy | No | No | Etanercept | Usual care |
| Semiglazov et al., 2006 115 | 25–55 | Breast cancer | On therapy | No | No | Mistletoe preparation | Placebo |
| Berk et al., 2008 116 | 23–91 | >1 Type | On therapy | No | No | β-Hydroxyl β-methyl butyrate (HMB), glutamine, and arginine | Isonitrogenous,isocaloric |
| Troger et al., 2009 117 | NR | Breast cancer | On therapy | No | No | Iscador M specialb | Usual care |
| Jeong et al., 2010 118 | NR | >1 Type | On therapy | No | Yes Bojungikki-tang (TJ-41) | Usual care | |
| Tian et al., 2010 119 | NR | Lung cancer | Off therapy | No | No | Feiji recipe | Usual care |
| Anthony et al., 2011 120 | NR | >1 Type | On therapy | No | No | Iron sucrose plus ESA | ESA |
| Barton et al., 2011 121 | NR | >1 Type | On therapy | No | No | Valerian | Placebo |
| Dimsdale et al., 2011 122 | NR | >1 Type | On therapy | Both | No | Eszopiclone | Placebo |
| Ikeguchi et al. 2011, 123 | NR | Colorectal cancer | On therapy | No | No | Fucoidan | Usual care |
| Chen et al., 2012 124 | NR | >1 Type | Both | No | Yes | Astragalus membranaceus | Placebo |
| Zhang et al. 2012, 125 | NR | Lung cancer | On therapy | No | No | Buckangling | Placebo |
| Del Fabbro et al., 2013 126 | NR | >1 Type | On therapy | No | No | Testosterone | Placebo |
| del Giglio et al., 2013 127 | NR | >1 Type | On therapy | No | Yes | Paullinia cupana | Placebo |
| Lesser et al., 2013 128 | 28–85 | Breast cancer | On therapy | No | No | Coenzyme Q10 | Placebo |
| Wen et al., 2013 129 | NR | >1 Type | On therapy | No | No | Thalidomide and megestrol acetate | Megestrol |
| Hansen et al., 2014 130 | 46–68 | Breast cancer | On therapy | No | No | Melatonin | Placebo |
| Hui et al., 2014 131 | 27–75 | >1 Type | On therapy | No | No | Fentanyl | Placebo |
| Law et al., 2014 132 | 30–73 | Breast cancer | On therapy | No | No | Virgin coconut oil | Usual care |
| Lee et al., 2014 133 | NR | Colorectal cancer | Off therapy | No | No | Probiotic preparation | Placebo |
| Sanchez-Lara et al., 2014 134 | NR | Lung cancer | On therapy | No | No | Eicosapentaenoic | Usual care |
| Terkawi et al., 2014 135 | NR | Breast cancer | On therapy | No | No | Lidocaine | Placebo |
| Wang et al., 2014 136 | NR | Lung cancer | On therapy | No | No | rHuBNP | Usual care |
| Liu et al., 2015 137 | 40–74 | >1 Type | On therapy | No | No | Olanzapine | Usual care |
| Birgegard et al., 2016 138 | 21–87 | >1 Type | On therapy | No | No | Iron isomaltoside | Iron sulphate |
| Jeon et al., 2016 139 | NR | Colon cancer | On therapy | No | No | Vitamin C | Placebo |
| Mofid et al. 2016, 140 | NR | >1 Type | On therapy | No | Yes | Royal jelly and honey | Honey |
| Faramarzi et al., 2017 141 | NR | Rectal cancer | On therapy | No | No | Conjugated linoleic acid | Placebo |
| Martins et al., 2017 142 | NR | Head-and-neck cancer | On therapy | No | No | Guarana | Placebo |
| Ribeiro et al., 2017 143 | NR | Colorectal cancer | Both | No | No | Zinc supplement | Placebo |
| Sun et al., 2017 144 | 18–90 | Gastric cancer | Off therapy | No | No | Jinlongshe granule | Placebo |
Studies included differing agents within Traditional Chinese Medicines.
Iscador Ltd., Lörrach, Germany.
HSCT = hematopoietic stem-cell transplantation; NR = not reported; SC = subcutaneous; rHuEPO = recombinant human erythropoietin; PO = oral; IV = intravenous; CTx = chemotherapy; TPN = total parenteral nutrition; ESA = erythropoiesis stimulating agents; IM = intramuscular; CFU = colony-forming units; rHuBNP = recombinant human B-type natriuretic peptide.