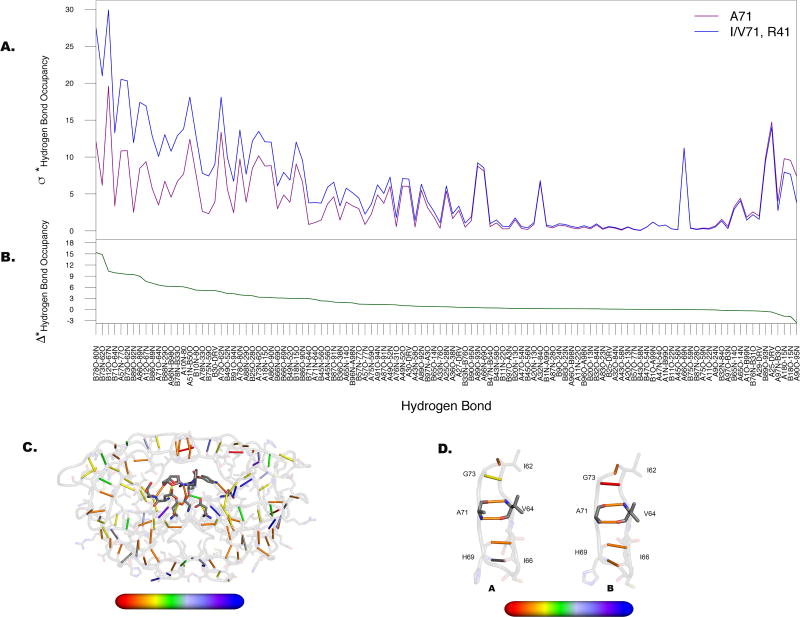
Figure 4. Dominant alterations in hydrogen bond occupancies emanate from lower cantilever region of the protease.
A. Departure from the mean (σ*) as calculated for the two groups in Figure 3A. Maximal separation between those variants lacking substitutions at position 71 (purple) and those containing substitutions at 71 (blue) occurs predominantly at hydrogen bonds formed with residues within the 70s β-strand. B. Difference between two lines in A (Δ*) with values plotted onto the structure C from red (high variability) to blue (low variability). D. Hydrogen bonds formed between residues surrounding A71 as labeled, for both monomers of the protease. Hydrogen bonds in this region have higher alterations in chain B than in chain A.