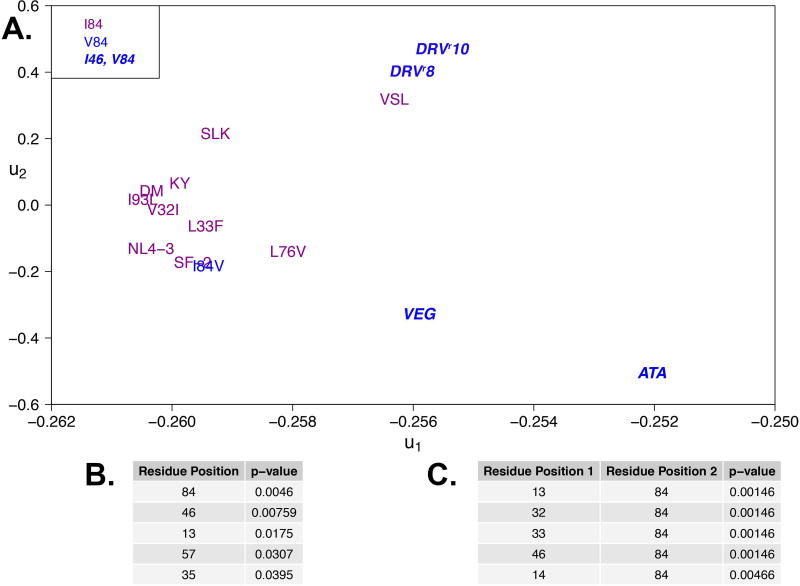
Figure 5. Ligand-protease van der Waals contact energies reveal energetic similarities between single site accessory RAMs and clinically-derived variants.
A. Fifteen sequence variants projected onto the first two principal components for the correlation matrix of 64 mean protease–DRV van der Waals contacts. Variants are partitioned into two groups, those bearing substitutions at positions 84 and/or 46 (blue) and those that do not (purple), similar to Figure 3A. B–C List of top single positions and paired positions most likely underlying changes in van der Waals contact energies.