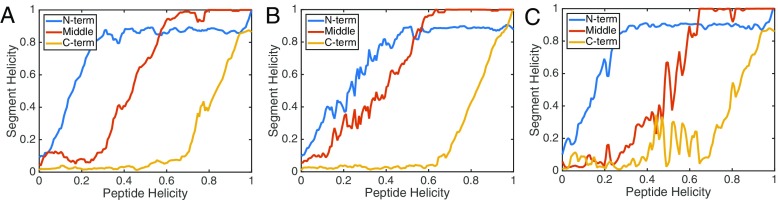
Fig. 3.
Segmental folding behavior of γ1 in PC (a), PG (b), and mixed (c) bilayers. The total peptide folding was dissected into three overlapping ten-residue segments: residues 1–10 (N-term), residues 6–15 (middle), and residues 12–21 (C-term). The helicity values are obtained by averaging the Sα values of the segments over the final 10 ns in each US window. The absolute Sα values are divided by the theoretical limit which is 5 for a ten-residue segment. Curves are smoothed by performing a five-point moving average