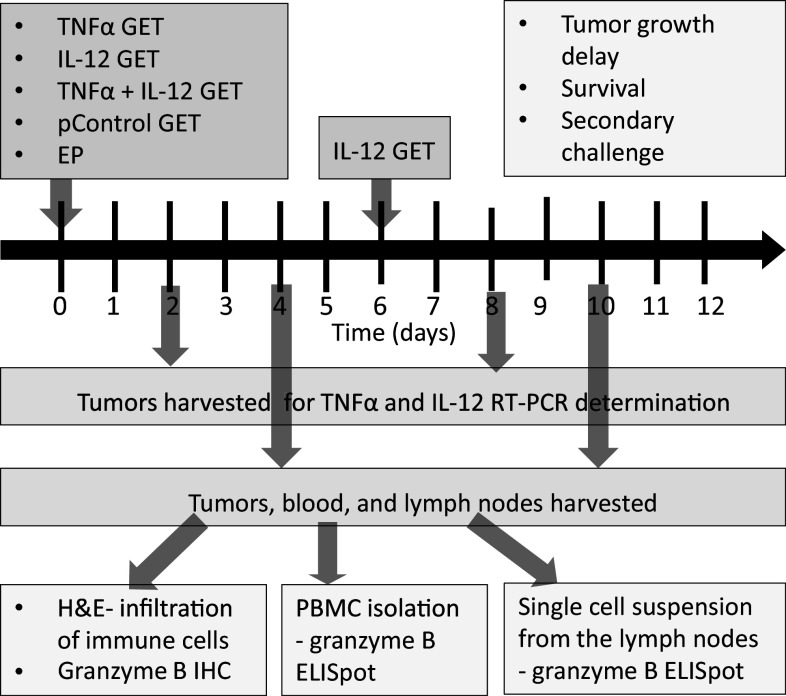
Fig. 1.
Experimental design. The tested therapeutic groups were as follows: TNF, gene electrotransfer (GET) of the TNFα plasmid; IL-12 GET, GET of the IL-12 plasmid repeated twice with an interval of 6 days; and TNF + IL-12, concomitant GET of the TNFα and IL-12 plasmids, followed 6 days later by GET of the IL-12 plasmid. Additional control groups were as follows: CTRL complete control, EP electroporation only, pControl GET of a control plasmid. On days 2 and 8 tumors were harvested for determination of TNFα and IL-12 expression by RT-PCR. Tumor growth was monitored until the tumor reached a volume of 300 mm3, which was also used as the endpoint event for plotting the Kaplan–Meier survival curve. Mice that were tumor free for 90 days were subjected to secondary challenge with an injection of tumor cells. Tumors, blood and lymph nodes were collected on days 4 and 10. Tumors were used for histological determination of immune cell infiltration (H&E, hematoxylin and eosin staining) and immunohistochemical (IHC) determination of granzyme B-positive cells. Granzyme B ELISpot was performed on the PBMC and single-cell suspensions isolated from the lymph nodes