Abstract
The outcome of small cell lung cancer (SCLC) patients is poor because rapid metastasis develops after first‐line chemotherapy and few drugs are available for second‐line chemotherapy. The median survival rate has not significantly changed in recent years. In this report, we discuss the case of a 71‐year‐old Chinese female non‐smoker diagnosed with extensive‐stage SCLC who was treated with nivolumab for a short period and obtained a prolonged clinical benefit. We report the clinical history, clinical features, potential mechanism, benefits, and the best therapeutic window. The patient was treated with transcatheter arterial chemoembolization because of liver metastasis and then with four doses of nivolumab as third‐line systemic treatment. There was no disease progression for 15 months. The lesions became larger than before, suggesting disease progression, thus nivolumab treatment was ceased. Immunotherapy has the capacity to turn combined therapy into a feature that may be exploited for clinical benefit. Further research is required to evaluate whether combined treatment is beneficial for patients, affecting the efficacy of immunotherapy, and to determine the best therapeutic window for clinical treatment.
Keywords: Cancer immunotherapy, nivolumab, small cell lung cancer, TACE
Introduction
Small cell lung cancer (SCLC), which accounts for approximately 15% of lung cancer cases, is an aggressive disease characterized by rapid growth and early widespread metastasis.1 The National Comprehensive Cancer Network (NCCN), the European Society for Medical Oncology (ESMO), and the American Society of Clinical Oncology (ASCO) recommend the use of chemotherapy with cisplatin plus etoposide (EP) to treat SCLC. Although SCLC is sensitive to chemotherapy (platinum‐etoposide) and radiotherapy, the outcome for SCLC patients is poor as rapid metastasis develops after first‐line chemotherapy and there are few drugs available for use as second‐line chemotherapy. In recent years, the median survival rate has not significantly changed and prognosis remains poor with a median survival of < 12 months for extensive disease (ED‐SCLC).2
Tumor immunotherapy is becoming a new treatment model for use after surgery, chemotherapy, radiotherapy, and target treatment. Cancer immunotherapy, which exploits the immunogenicity of tumor antigens, improves the ability of the immune system to recognize and kill tumor cells. Chen and Mellman detailed the cancer‐immunity cycle, indicating that cellular alterations provide the immune system with the means to generate T cell responses that recognize and eliminate cancer cells.3 The cancer‐immunity cycle contains several important steps: (i) the release of cancer cell antigens (cancer cell death); (ii) cancer antigen presentation (dendritic cells and antigen‐presenting cells [APCs]); (iii) priming and activation (APCs and T cells); (iv) trafficking of T cells to tumors (cytotoxic T lymphatic cells [CTLs]); (v) infiltration of T cells into tumors (CTLs, endothelial cells); (vi) recognition of cancer cells by T cells (CTLs, cancer cells); and (vii) death of cancer cells (immune and cancer cells). According to the mechanisms of different drugs, we know that cytotoxic drugs or radioactive rays can result in cancer cell death, which reduces the release of cancer cell antigens; vaccines can carry cancer antigens or proteins that play an anti‐tumor role vis CTL; and some anti‐VEGFs withstand infiltration of T cells into tumors and anti‐PD‐1 to kill cancer cells. Emerging clinical data has shown that cancer immunotherapy is likely to become a key target for the clinical management of cancer.
Effective molecular targets are the key to tumor immunotherapy. PD‐1, one of the newer pathways in the B7:CD28 family, is a 288 amino acid type I transmembrane protein composed of one immunoglobulin superfamily domain, expressed at the surface of activated CD4+/CD8+ T, B, natural killer, mononuclear, and dendritic cells as monomers. PD‐L1 (B7‐H1) in the B7 superfamily is the most common ligand of PD‐1, which is expressed in immune cells (e.g. tumor infiltrating lymphocytes) and epithelial cells (e.g. tumor cells).4, 5 Monoclonal antibodies (Ig4 mAb) pembrolizumab (MK‐3475) and nivolumab (MDX‐1106), certified by the United States Food and Drug Administration, block interaction of PD‐1 at the T cell surface and PD‐L1/PD‐L2 in tumor cells; the drugs bind PD‐1 to elicit a specific cellular immune response. Research has shown that one or more phases of therapy could cease disease progression and induce long‐term immune control. Pembrolizumab and nivolumab have been used in large number of published clinical trials (CheckMate‐017, CheckMate057, KEYNOTE‐010, POPLAR),6, 7, 8 and were included in the 2016 NCCN guidelines for standard second‐line treatment of non‐small cell lung cancer (NSCLC).9 The CheckMate 032 study reported that nivolumab alone or in combination with ipilimumab possesses antineoplastic activity that could safely control recurrent SCLC.10
Case presentation
Herein, we report a case of a 71‐year‐old female non‐smoker with no cancer history. She was diagnosed with ED‐SCLC (left small pleural effusion) in August 2015. Tracheoscopic biopsies revealed small cell carcinoma, and positron emission tomography‐computed tomography (PET‐CT) showed no evidence of metastatic disease; however, liver metastasis was suspected. Treatment with thoracic irradiation followed by first‐line systemic chemotherapy was planned. She was treated with four cycles of first‐line systemic chemotherapy with lobaplatin 25 mg/m2 intravenously (iv) on day 1 and vincristine 100 mg/m2 iv on days 1–3. After four cycles of chemotherapy, the patient achieved partial remission. As a result of grade 3 myelosuppression caused by chemotherapy, the patient was too weak to treat further. However, disease progression with significantly larger lung lesions was observed a month after first‐line chemotherapy was completed, thus thoracic irradiation was commenced in December 2015. The patient was regularly followed‐up every two months. She received two cycles of second‐line chemotherapy with irinotecan in April 2016 after a new lesion was discovered in her liver. In June 2016, she underwent abdominal magnetic resonance imaging, which revealed a lesion in the right lobe of the liver measuring 8.4 x 6.0 x 7.1 cm (Fig 1), and chest CT showed enlarged mediastinal lymph nodes. She was treated with transcatheter arterial chemoembolization (TACE) for the liver metastases and chemotherapy of vincristine 100 mg iv on days 1–5. TACE is a process of drug delivery and embolization performed via transfemoral intervention that allows access to the hepatic artery, after which tumor size, number, and staining are observable on CT. Treatment included 2012 Fahrenheit iodinated oil 25 mL, cisplatin 60 mL; however, the patient developed grade 4 myelosuppression. T lymphocyte cell subsets in the peripheral blood were measured by flow cytometry before and after surgery. CD3, CD4, and CD4/CD8 levels (but not CD8), increased after surgery. Immunotherapy was applied as third‐line systemic therapy. Nivolumab was initiated at a dose of 3 mg/kg iv every two weeks prior to molecular characterization of the tumor. After three doses, radiographic imaging showed a decrease in the size of all target lesions and slight thickening of the left adrenal gland (Fig 2). Concurrently, all cell counts began to decrease. Radiographic imaging showed that the liver lesion continued to decrease and the lung lesions became stable after four weeks and four doses of nivolumab (Fig 1). The patient was followed‐up every five to six months. Clinical remission was achieved for 15 months, and was attributed to nivolumab treatment. However, progressive disease was observed in October 2017 after the lesions in the left adrenal gland and mediastinal lymph node significantly increased (Fig 3). The patient is currently undergoing adrenal and mediastinal lymph node radiotherapy.
Figure 1.
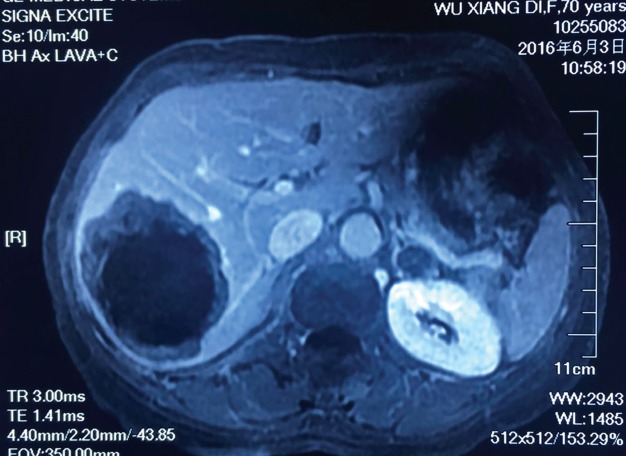
Abdominal magnetic resonance imaging taken in June 2016 revealed a lesion in the right lobe of the liver measuring 8.4 x 6.0 x 7.1 cm.
Figure 2.

Summary of treatment and tumor response. (a) Various treatments administered. Arrowheads indicate time points for each intervention. (b) (i–v) Chest or abdominal computed tomography images of the lung, mediastinal lymph nodes, and liver lesions before and after nivolumab treatment. Each lesion had been in remission. The metastatic liver lesion retreated. Subsequent computed tomography scans showed stable disease.
Figure 3.
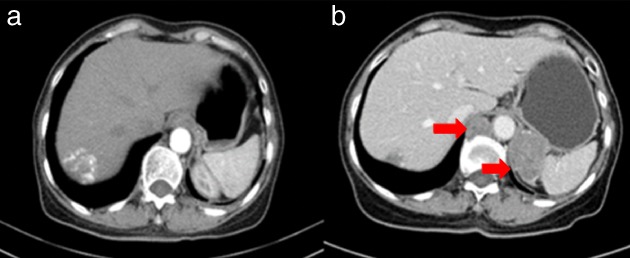
Lesions in the left adrenal gland and mediastinal lymph nodes: (a) May 2017; (b) October 2017.
Discussion
We report the case of a patient with metastatic SCLC who experienced 15 months of clinical remission after two months of nivolumab treatment. From a therapeutic standpoint, 70–80% of limited stage SCLC (LS‐CLC) and almost all ED‐SCLC cases exhibit disease progression or/and recurrence within months after commencing traditional chemotherapy.11 The patient in our case underwent traditional chemoradiotherapy and received five months of progression‐free survival (PFS) after first‐line treatment and a further two months PFS after second‐line treatment, similar to the results of previous studies.11 However, the patient obtained a clinical benefit from four‐cycles of nivolumab.
The results of this case indicate that the combination of immunotherapy and TACE is a key feature for predicting response to treatment. The main mechanism of TACE to treat metastatic hepatic carcinoma is the application of different embolic agents to block the blood supply to tumor cells. Anastomosis between the hepatic artery and the portal veins causes a compensatory venous blood increase once blood supply to the tumor cells is blocked. New collateral and anastomosis vessel formation increases tumor growth after TACE. Furthermore, a vicious cycle between tumor growth and increased blood vessel formation develops. Recent evidence suggests a tumor necrosis rate of approximately 10–20% after TACE.12, 13 It has been suggested that as tumor cells metastasize, angiogenesis of residual tumor cells occurs.14 The outcome after using TACE alone is poor but in combination with immunity treatment is beneficial, enhancing the therapeutic effect.
Tumor immunity is mainly associated with the cellular immunity of T lymphocytes, an indicator of cellular immunity function. T cells contain CD4+ helper lymphocyte/inducer lymphocyte T cells and CD8+ exhausted/cytotoxic lymphatic T cells according to surface marker and function. CD4+ T cells are T helper lymphocytes (Th), which primarily mediate specific cellular immune function. Activated CD8+ T cells are known as CTLs. Th can activate CTLs, which can in turn identify tumor antigens to kill tumor cells directly. CTLs also secrete lymphatic factors and mediate macrophage activation. The combination of PD‐1 and PD‐L1 can suppress CTL proliferation to weaken the anti‐tumor effect and reduce the secretion of IL‐2 to weaken T cell activation.15, 16 Nivolumab generates CTL activation to promote the proliferation of T cells.17 Th is activated by tumor cells to generate lymphatic cell invasion and dual signal stimulation by some surface molecules. The CD4+:CD8+ T cell ratio in peripheral blood is a sensitive indicator of immune balance.17
In our study, numbers of CD3+, CD4+, and CD4+/CD8+ T cells were significantly higher after surgery, similar to the results of previous reports. After TACE, the number of CD4+ T cells increased, while CD8+ T cells decreased. Th causes a reduction in the number CD8+ T‐cells. Nivolumab can influence PD‐1 and increase CD4+ and CD8+ T cell proliferation, particularly CD8+ T‐cell proliferation.18 Nivolumab increases CD8+ T cells in the tumor microenvironment and may decrease these in peripheral blood. Therefore, TACE combined with nivolumab may increase the cellular immune response, promoting the synergistic death of tumor cells. An increase in the number of CD4+ T cells can increase the number and function of CD8+ T cells in the tumor microenvironment after TACE, providing continuous clinical benefit. In addition, TACE can block blood, which cancer tumor cells, and in turn reduces the release of cancer antigens and CD8+ T cells that can infiltrate the tumor microenvironment. An increase in the number of CD4+ T cell activates CTL to enhance cancer immunity. The results indicate that TACE can improve the cellular immune state of patients to allow clinical remission.
Cisplatin may be another reason for remission. Researchers have reported that cisplatin can enhance cytotoxic effects to kill tumor cells and impact immunity. Parikh et al. discovered PD‐L1 overexpression at cell surface after cisplatin treatment in hepatocellular carcinoma cell lines.19 PD‐L1 combined with PD‐1 provided inhibitory signals to depress T and B cell differentiation and appreciation and reduced the anti‐tumor effect. Cisplatin plus immune therapy decreases PD‐L1 expression at the cell surface and increases CD8+ T cells, which reverses cancer cell tolerance. The presence of suppressive factors in the tumor microenvironment may explain why these therapies may be more effective in combination with agents that target other steps of the cycle.3
Immunotherapy combined with chemotherapy, radiotherapy or other immune therapies has demonstrated a synergistic effect and clinical benefits. Checkmate‐032 interim analysis showed that nivolumab alone or in combination with ipilimumab controlled disease progression after at least one previous platinum‐based regimen.10 Patients who received nivolumab alone or nivolumab plus ipilimumab achieved overall response rates of 33%, 23%, 19%, and 10%. CheckMate 012 showed that nivolumab combined with platinum‐based chemotherapy as first‐line treatment for in advanced NSCLC improved the overall survival rate (OS).20 Two year OS after combination treatment of nivolumab 5 mg/kg and paclitaxel plus carboplatin was 62%; however, this treatment is accompanied by a greater risk of adverse reactions.
Radiation can induce immune‐related phenotypic changes of cells in the microenvironment, which may induce the anti‐tumor immune response. Four retrospective studies compared stereotactic radiotherapy combined immunotherapy in patients with malignant melanoma brain metastases.21 Two of the studies found that combined therapy can significantly prolong median survival, while the other two studies did not reach this conclusion. All existing retrospective studies were small and research continues to confirm these results. There is no data assessing the outcome of TACE combined with immune treatment. PD‐1 combined with chemotherapy, radiotherapy, and immune therapy can achieve a clinical benefit and longer PFS and OS. Further research is required to explore the mechanisms and clinical outcomes of combined therapy.
Phase III clinical studies of the use of nivolumab in squamous (CheckMate017; NCT01642004) and non‐squamous (CheckMate057; NCT01673867) NSCLC have shown that nivolumab can prolong patient survival compared to docetaxel.22 The two‐year OS rates in the squamous NSCLC group were 23% (95% confidence interval [CI] 16–30%) for nivolumab and 8% (95% CI 4–13%) for docetaxel, while in the non‐squamous NSCLC group the rates were 29% (95% CI 24–34%) and 16% (95% CI, 12–20%), respectively. In the squamous and non‐squamous NSCLC groups, the nivolumab treatment group had two‐year PFS rates of 16% (95% CI 10–23%) and 12% (95% CI 8–16%), respectively. Further research is required to detect optimal PD‐1 and combined treatment windows.
Unlike the more common histological types of lung cancer, PFS is extremely short for SCLC, even when diagnosed at an early stage. Resistance to traditional treatment, reflected in the heterogeneity of driver mutations and sensitivity to treatment described in the literature, encourages oncologists to look for novel therapies. Immunotherapy has the capacity to turn combined therapy into a feature that may be exploited for clinical benefit. TACE with nivolumab can interfere with several steps of the cancer immunity cycle to enhance anti‐tumor effect. Exploration of combined immune therapy compared to targeted treatment is complicated. The biological nature of tumor and immune cells needs to be considered simultaneously in order to explore the effect of combined immunotherapy.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported by the Beijing Municipal Science and Technology Commission (No. 171100001017127) and the Fund for National Natural Science Foundation of China (Grant 81472186).
[Correction added on 1 May 2018, after first online publication: ‘Peking University’ has been removed from the affiliation address and Jun Liang's correspondence.]
Contributor Information
Chuanhao Tang, Email: gallanttang@126.com.
Jun Liang, Email: liangjun1959@aliyun.com.
References
- 1. Alvarado‐Luna G, Morales‐Espinosa D. Treatment for small cell lung cancer, where are we now? A review. Transl Lung Cancer Res 2016; 5: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaspar LE, McNamara EJ, Gay EG et al. Small cell lung cancer: Prognostic factors and changing treatment over 15 years. Clin Lung Cancer 2012;13:115–22. [DOI] [PubMed] [Google Scholar]
- 3. Chen DS, Mellman I. Oncology meets immunology: The cancer‐immunity cycle. Immunity 2013; 39: 1–10. [DOI] [PubMed] [Google Scholar]
- 4. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller JF, Sadelain M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell 2015; 27: 439–49. [DOI] [PubMed] [Google Scholar]
- 6. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garon EB, Rizvi NA, Hui R et al Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 9. Ettinger DS, Wood DE, Akerley W et al NCCN guidelines insights: Non‐small cell lung cancer, version 4.2016. J Natl Compr Canc Netw 2016; 14: 255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antonia SJ, López‐Martin JA, Bendell J et al Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): A multicentre, open‐label, phase1/2 trail. Lancet Oncol 2016; 17: 883–95. [DOI] [PubMed] [Google Scholar]
- 11. Garassino MC, Torri V, Michetti G et al Outcomes of small‐cell lung cancer patients treated with second‐line chemotherapy: A multi‐institutional retrospective analysis. Lung Cancer 2011; 72: 378–83. [DOI] [PubMed] [Google Scholar]
- 12. Burrel M, Reig M, Forner A et al Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolization (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol 2012; 56: 1330–5. [DOI] [PubMed] [Google Scholar]
- 13. Kawaguchi T, Ohkawa K, Imanaka K et al Lipiodol accumulation and transarterial chemoembolization efficacy for HCC patients. Hepatogastroenterology 2012; 59: 219–23. [DOI] [PubMed] [Google Scholar]
- 14. Liu JW, Yi JL. The effect of transcatheter arterial chemoembolization on dendritic cells in hepatocellular carcinoma. Chin J Gen Surg 2007; 16: 209–12. [Google Scholar]
- 15. Blank C, Mackensen A. Contribution of the PD‐L1/PD‐1 pathway to T‐cell exhaustion: An update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007; 56: 739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carter L, Fouser LA, Jussif J et al PD‐1:PD‐L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL‐2. Eur J Immunol 2002; 32: 634–43. [DOI] [PubMed] [Google Scholar]
- 17. Yasutomo K. The cellular and molecular mechanism of CD4/CD8 lineage commitment. J Med Invest 2002; 49: 1–6. [PubMed] [Google Scholar]
- 18. Kilinc MO, Aulakh KS, Nair RE et al Reversing tumor immune suppression with intratumoral IL‐12: Activation of tumor‐associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol 2006; 177: 6962–73. [DOI] [PubMed] [Google Scholar]
- 19. Parikh F, Duluc D, Imai N et al Chemoradiotherapy‐induced upregulation of PD‐1 antagonizes immunity to HPV‐related oropharyngeal cancer. Cancer Res 2014; 74: 7205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hellmann MD, Rizvi NA, Goldman JW et al Nivolumab plus ipilimumab as first‐line treatment for advanced non‐small‐cell lung cancer (CheckMate 012): Results of an open‐label, phase 1, multicohort study. Lancet Oncol 2017; 18: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tallet AV, Dhermain F, Le Rhun E, Noel G, Kirovs YM. Combined irradiation and targeted therapy or immune checkpoint blockade in brain metastases: Toxicities and efficacy. Ann Oncol 2017; 28: 2962–76. [DOI] [PubMed] [Google Scholar]
- 22. Horn L, Spigel DR, Vokes EE et al Nivolumab versus docetaxel in previously treated patients with advanced non‐small‐cell lung cancer: Two‐year outcomes from two randomized, open‐label, phase III trials (CheckMate017 and CheckMate 057). J Clin Oncol 2017; 35: 3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


