Figure 7.
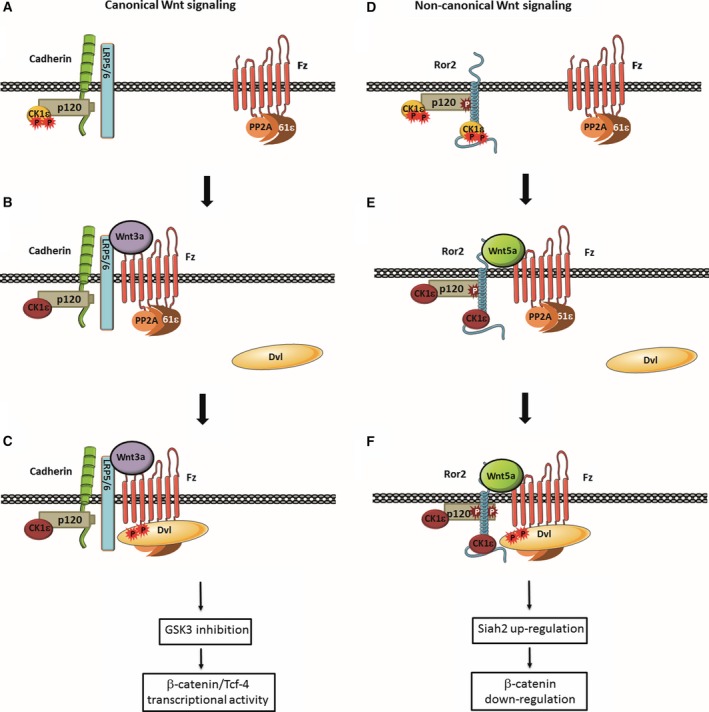
Model for the initiation of Ror2‐dependent noncanonical Wnt signaling. In the canonical Wnt pathway, Ser‐phosphorylated and inactive CK1ε (orange) binds to the LRP5/6 co‐receptor through cadherin and p120‐catenin (A); upon Wnt3a stimulation, PP2A, associated with Fz through the PR61ε regulatory subunit, becomes closer to LRP5/6‐bound CK1ε, allowing it to dephosphorylate and thus activate this kinase (B). Active CK1ε (dark red) phosphorylates either Dvl2, Fz, or both, thereby facilitating the interaction of Dvl2 with the receptor complex (C) and enabling the further reactions of this pathway, which lead to GSK3 inactivation and stimulation of β‐catenin/TCF‐4 transcriptional activity. Wnt5a activates noncanonical signaling using the same receptor but a different co‐receptor – namely, Ror2, which interacts with CK1ε both directly and indirectly through p120‐catenin (D). Association with p120‐catenin requires tyrosine phosphorylation of this protein and protects Ror2 from clathrin‐mediated internalization. Similar to the canonical Wnt pathway, Wnt5a‐induced assembly of the Fz–Ror2 complex enables CK1ε dephosphorylation and activation by PP2A (E), recruitment of Dvl2 to the complex (F) and an increase of p120‐catenin Tyr phosphorylation, which enhances its interaction with Ror2. Dvl2 binding to the complex is required for downstream signaling events, such as Rac1 and JNK activation, Siah2 expression, and β‐catenin downregulation.
